 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) Give the name of reagent which when reacted with concentrated hydrochloric acid produce chlorine gas.
(b) A student set out to prepare iron (III) chloride using the apparatus shown in the diagram below.
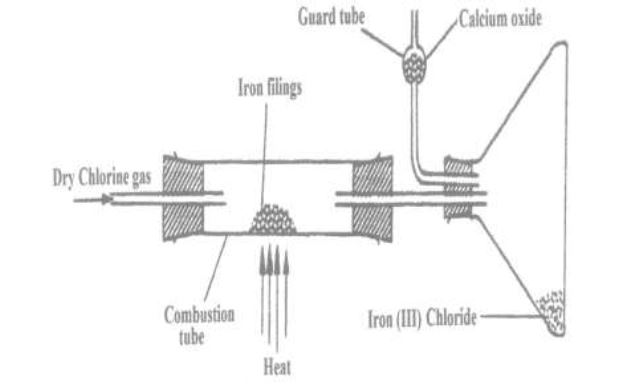
(i) Explain why:
(I) It is necessary to pass chlorine gas through the apparatus before heating begins.
(II) Calcium oxide would be preferred to calcium chloride in the guard tube.
(ii) What property of iron (III) chloride makes it possible to be collected as shown in the diagram?
(iii) Write an equation for one chemical reaction that took place in the guard tube.
(iv) The total mass of iron (III) chloride formed was found to be 0.5g.
Calculate the volume of chlorine gas that reacted with iron. (Fe = 56.0, CI = 35.5 and molar gas volume at 298K is 24,000#cm^3#).