 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) The table below shows some properties of chlorine, bromine and iodine.
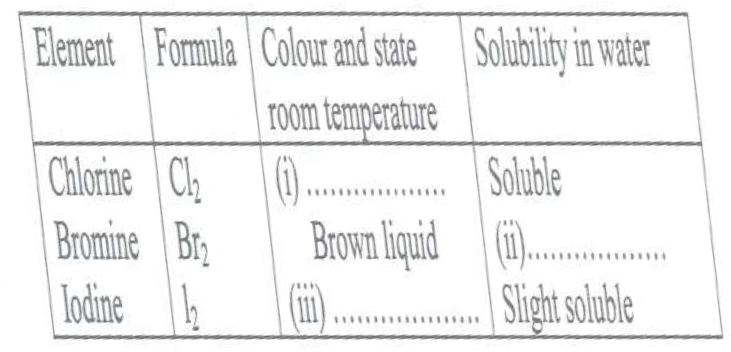
Complete the table by giving the missing information in (i), (ii) and (iii).
(b) Chlorine gas is prepared by reacting concentrated hydrochloric acid with manganese (IV) oxide.
(i) Write the equation for the reaction between concentrated hydrochloric acid and manganese (IV) oxide.
(ii) What is the role of manganese (IV) oxide in this reaction?
(c) (i) Iron (II) chloride reacts with chlorine gas to form substance E. Identify substance E.
(ii) During the reaction in c (i) above, 6.30g of iron (II) chloride were converted to 8.06g of substance E. calculate the volume of chloride gas used.
(CI=35.5, molar gas volume at room temperature =24000#cm^3#, Fe =56)
(d) Draw and name the structure of the compound formed when excess chlorine has is reacted with ethane gas.
(e) Give the industrial use of chlorine.