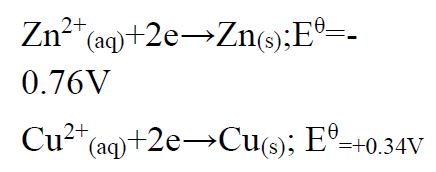Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a)In an experiment to determine the molar heat of reaction when magnesium displaces copper, 0.15 g of magnesium powder were added to #25.0cm^3# of 2.0M copper (II) chloride solution. The temperature of copper (II) chloride solution was #25^0C#,while that of the mixture was #43^0C#.
(i) Other than increase in temperature, state and explain the observations which were made during the reaction.
(ii)Calculate the heat change during the reaction (specific heat capacity of the solution=#4.2jg^-1K^-1 #and the density of the solution=1g/#cm^3#
(iii) Determine the molar heat of displacement of copper by magnesium (Mg=24.0)
(iv) Write the ionic equation for the reaction.
(v) Sketch an energy level diagram for the reaction.
(b)Use the reduction potentials given below to explain why a solution containing copper ions should not be stored in a container made of zinc.