 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a)The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.
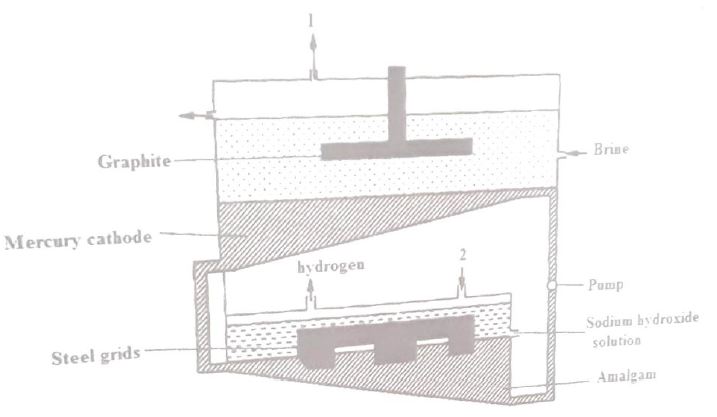
(i) Name
I.the raw material introduced at 2.
II.another substance that can be used in the cell instead of graphite.
(ii) Identify the by-product that comes out at 1.
(iii) Give
I.one use of sodium hydroxide.
II.two reasons why mercury is recycled.
(b) A current of 100 amperes was passed through the cell for five hours.
(i) Write the equation for the reaction that occurred at the mercury cathode.
(ii) Calculate the mass of sodium hydroxide that was produced.
(Na=23.0, O=16.0, H=1.0, 1 Faraday =96500 Coulombs)