 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
The table below gives standard electrode potentials for the metals represented by the letters D,E, F and G. Study it and answer the questions that follow.
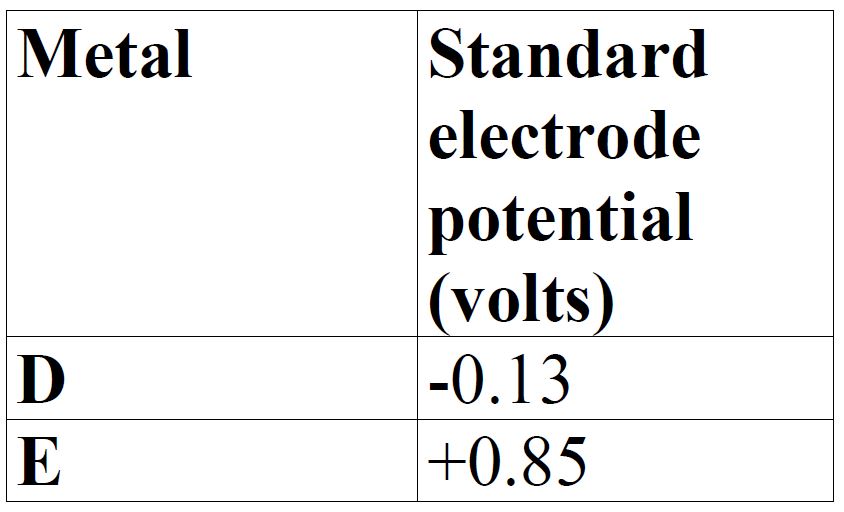
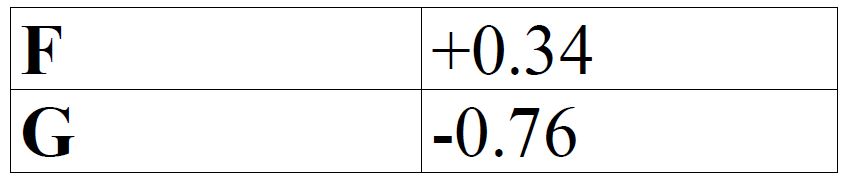
(a) Which metal can be displaced from a solution of its salt by all the other metals in the table? Give a reason.
(b) Metals F and G were connected to form a cell
as shown in the diagram below.
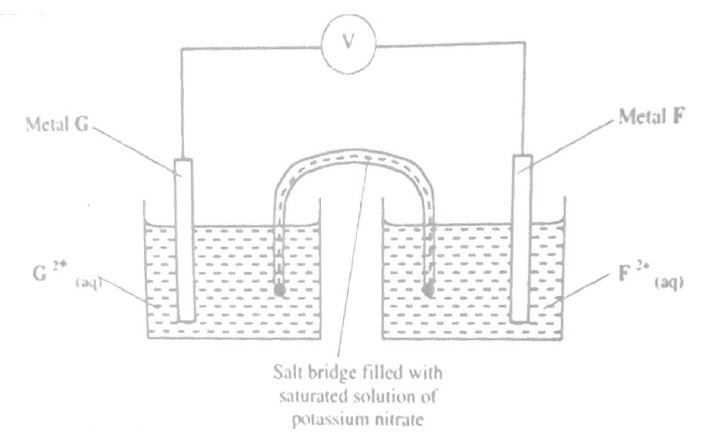
(i) Write the equation for the reactions that occur at electrodes F and G
(ii) On the diagram, indicate with an arrow the direction in which electrons would flow.
(iii) What is the function of the salt bridge?
.