 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Study the standard electrode potentials in the table below and answer the questions that follow.
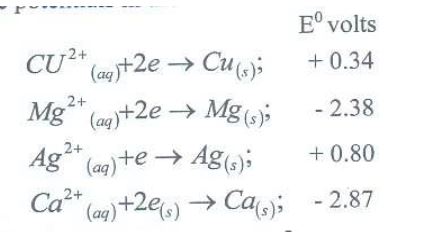
(a) Which of the metals is the strongest reducing agent?
(b) What observations will be made if a silver coin was dropped into an aqueous solution of copper (II) sulphate? Explain.