 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
The set-up below was used by a student to investigate the products formed when aqueous copper (II) chloride was electrolyzed using carbon electrodes.
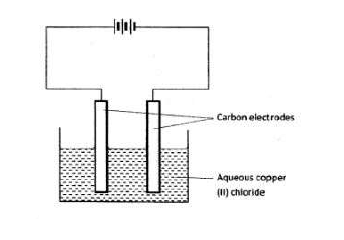
(a) (i) Write the equation for the reaction that takes place at the cathode.
(ii) Name and describe a chemical test for the product initially formed at the anode when a highly concentrated
solution of copper (II) chloride is electrolyzed.
(iii) How would the mass of the anode change if the carbon anode was replaced with copper metal? Explain.
(b) 0.6 g of metal B Were deposited when a current of O.45A was passed through an electrolyte for 72 minutes. Determine the charge on the ion of metal B. (Relative atomic mass of B = 59, 1 Faraday = 96 500 coulombs)
(c) The electrode potentials for cadmium and zinc are given below:
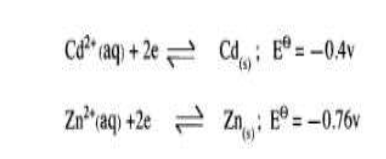
Explain why it is not advisable to store a solution of cadmium nitrate in a container made of zinc.