 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) The table below shows the standard reduction potentials for four half-cells. Study it and answer the questions that follow. (Letters are not the actual symbols of the elements)
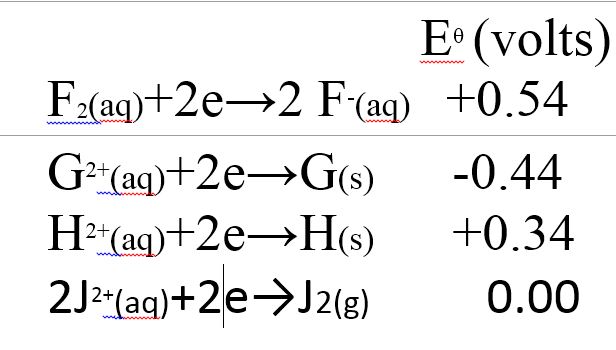
(i) Identify the strongest reducing agent.
(ii) Write the equation for the reaction which takes place when solid G is added to a solution containing #H^(2+)# ions.
(iii) Calculate the #E^θ# value for the reaction in (ii) above.
(b) The diagram below shows the apparatus that can be used to electrolyze acidified water to obtain hydrogen and oxygen gases. Study it and answer the questions that follow.
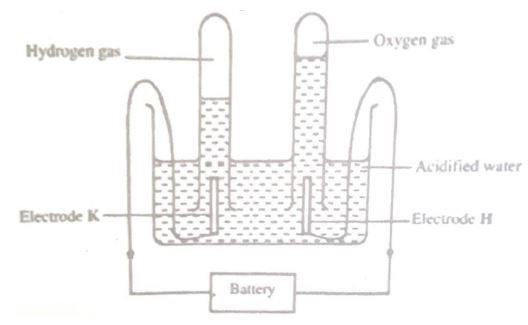
(i) Identify the electrode at which oxidation takes place.
(ii) Give a reason why it is necessary to acidify the water.