 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) Fractional distillation of liquid air usually produces nitrogen and oxygen as the major products.
(i) Name one substance that is used to remove carbon (IV) oxide from the air before
it is changed into liquid.
(ii) Describe how nitrogen gas is obtained from the liquid air.
(Boiling points nitrogen= -1960 C, oxygen= -1830 C).
(b) Study the flow chart below and answer the following questions.
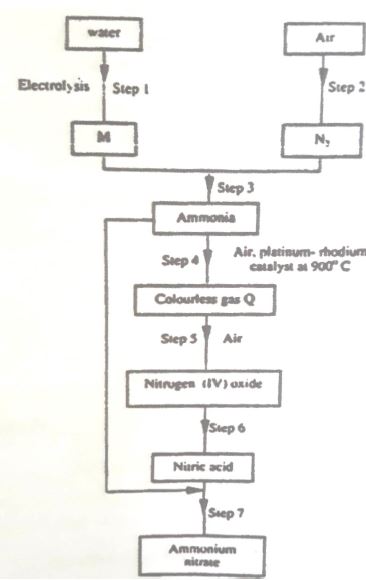
(i) Name element M.
(ii) Why is it necessary to use excess air in step 4?
(iii) Identify gas Q.
(iv) Write an equation for the reaction in step 7.
(v) Give one use of ammonia nitrate.
(c) State and explain the observations that would be made if a sample of sulphur is heated with concentrated nitric (V) acid.