 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) The schematic diagram below shows part of the Solvay process used for the manufacture of sodium carbonate.
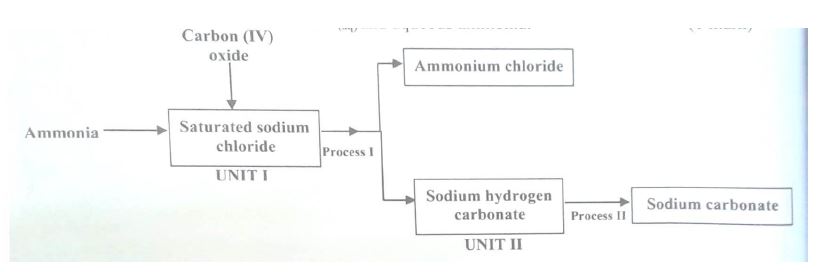
(i) Explain how the sodium chloride required for this process is obtained from sea water.
(ii) Two main reactions take place in UNIT I The first one is the
formation of ammonium hydrogen carbonate.
(I) Write an equation for this reaction
(II) Write an equation for the second reaction.
(iii) State how the following are carried out:
(I) Process I
(II) Process II
(iv) In an experiment to determine the percentage purity of the sample of sodium carbonate produced in the Solvay process, 2.15g of the sample reacted completely with 40.0#cm^3# of 0.5 M sulphuric (VI) acid.
(I) Calculate the number of moles of sodium carbonate that reacted.
(II) Determine the percentage of sodium carbonate in the sample.
(Na=23.0, C=12.0, O=16.0)
(b) Name two industrial uses of sodium carbonate.