 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) The diagram below shows a set-up that can be used to obtain nitrogen gas in an experiment.
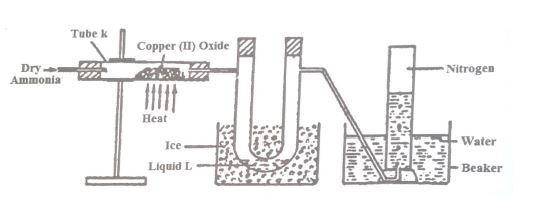
(i) Name liquid L.
(ii) What observation would be made in tube K after heating for some time?
(iii) Write an equation for the reaction that took place in tube K.
(iv) If 320 #cm^3# of ammonia gas reacted completely with the copper, calculate:n
(I) Volume of nitrogen gas provided.
(II) The mass of copper oxide that reacted.
(Cu = 63.5, O = 16.0, one mole of gas occupies 24 litres at room temperature and pressure).
(v) At the end of experiment, the pH of the water in the beaker was found to be about 10. Explain.
(b) In another experiment, a gas jar containing ammonia was inverted over a burning splint. What observation would be made?
(c) Why is it advisable to obtain nitrogen from air instead of from ammonia?