 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) In the flow chart below, some processes have been identified and others labelled. Study it and answer the questions that follow.
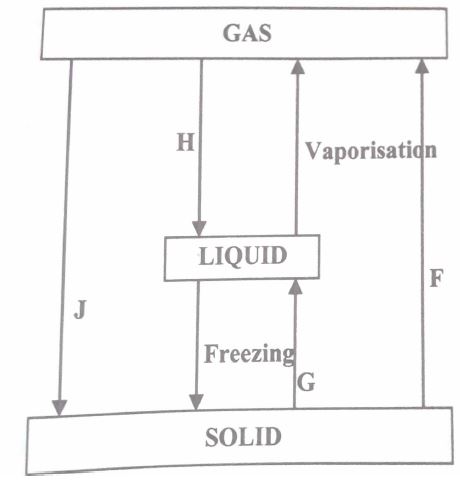
(i) Give the names of the processes:
I.H
II.G
(ii) Name one substance that can undergo process F when left in an open container in the laboratory.
(iii) The process J is called deposition. Using water as an example, write an equation that represents the process of deposition.
(b) The figure below shows the heating curve for water.
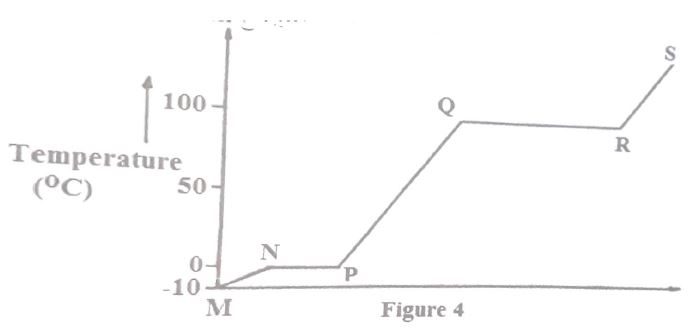
(i) Give the names of the intermolecular forces of attraction in the segments;
I.MN
II.RS
(ii) The heats of fusion and vaporization of water are 334.4J#g^-1# and 1159.4J#g^-1# respectively.
I. Explain why there is a big difference between the two.
II. How is the difference reflected in the curve?