 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) Distinguish between exothermic and endothermic reaction.
(b) Changes of state are either exothermic or endothermic. Name a change of state that is:
(i)Endothermic
(ii) Exothermic
(c ) When pure water is heated at 1 atmospheric pressure at sea level, the temperature of the water does not rise beyond #100^oC#,even with continued heating. Explain this observation.
(d)Study the energy cycle diagram below and answer the questions that follow:
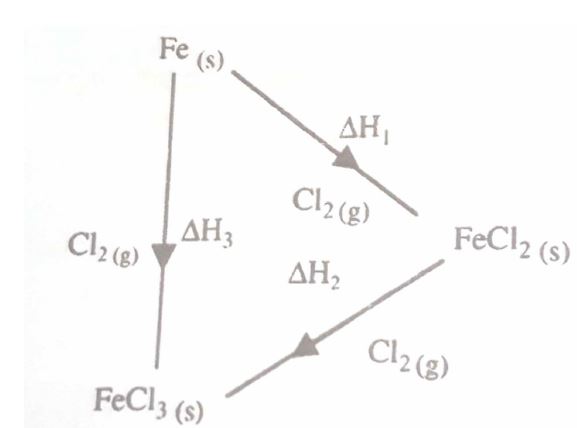
(i) What does #Delta#H1 represent?
(ii) Show the relationship between #Delta#H1, #Delta#H2 and #Delta#H3.
(e)Butane and propane are constituents of cooking gas. Which one produces more energy per mole on combustion? Explain.