 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 3 Chemistry Paper 1 End of Term 3 Exams 2021
Class: Form 3
Subject: Chemistry
Level: High School
Exam Category: Form 3 End Term 3 Exams
Document Type: Pdf
Views: 1035
Downloads: 26
Exam Summary
Answer all the questions in the spaces provided.
1. Three pure pigments were prepared and their spots placed on a filter paper as shown below. The
three pigments are A, B and C. A mixture F was also placed on the filter paper at the same time
with the pure pigments. The filter paper was then dipped in ethanol solvent and left for some half
an hour. The results were obtained as follows.
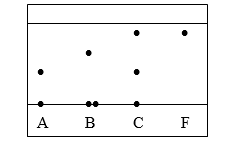
(i) Which of the three pure pigments is most sticky? Give a reason for your answer. (1mk)
(ii) Which pure pigment is not present in the mixture F? (1mk)
(iii) Show on the diagram the baseline. (1mk)
2. Describe how a pure sample of lead (II) carbonate can be prepared in the laboratory starting with lead II oxide. (3mks)
3. The set up below was used to prepare nitric (V) acid in the laboratory. (4mks)
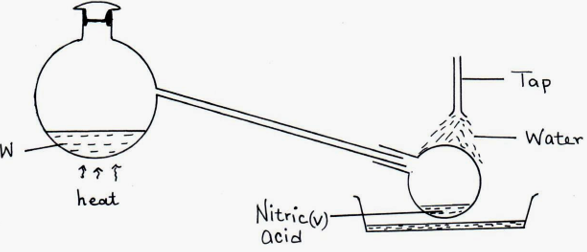
(a) Name the mixture W. (1mk)
(b) Write an equation for the reaction that takes place in flask A. (1mk)
(c)Explain why concentrated nitric (V) acid produced appears yellow when exposed to sun light (1mk)
4. A mixture contains ammonium chloride, aluminium oxide and sodium chloride. Describe how each solid substance can be obtained from the mixture. (3mks)
5. State the difference between the following salts;
Deliquescent and hygroscopic salts. (2mks)
6. Below is a set-up of apparatus used to investigate the effect of electric current on molten lead (II) bromide. (3mks)
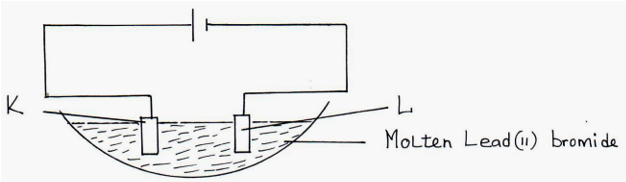
(a). Name electrodes (1mk)
(b). State the observation made at electrode K. (1mk)
7. A sample of a polymer has the following structure. (2mks)
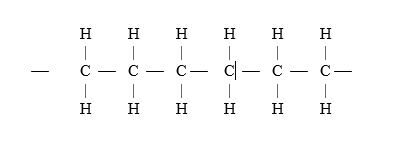
a) Draw the structural formula of the monomer that makes the above polymer. (1mk)
b) The polymer is found to have a molecular mass of 2268g. Determine the number of monomers in the polymer. (H = 1., C = 12). (1mk)
8. Study the information given in the table below and answer the questions that follows.
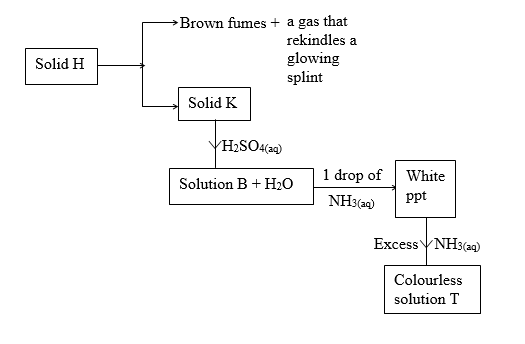
(a) Predict the cation and anion present, in solid H.
(b) Identify solid K, solution B and white-precipitate
Solid K (1mk)
Solution B (1mk)
White precipitate(ppt) (1mk)
9. The isotopes hydrogen are H and H. Determine the molecular masses of the molecules formed when each of these isotopes react with chlorine. (Cl = 37, H=1) (2mks)
10. The table below gives the atomic numbers of elements W,X,Y and Z. The letters do not represent the. actual symbol of the elements
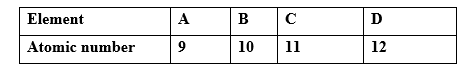
a) Which one of the elements is unreactive? Explain (1mk)
b)i) Which two elements would react most vigorously with each other? (1mk)
ii)Give the formula of the compound formed when the elements in b (i) above react (1mk)
11a) Distinguish between a hydrogen bond and covalent bond (2mks)
b) Explain why the boiling point of water is higher than that of hydrogen Sulphide
(Relative molecular mass of water is 18 while that hydrogen sulphide is 34) (2mks)
12. In an attempt to investigate the properties of halogens, a student bubbled chlorine gas through a solution of potassium bromide.
(a)State and explain what was observed. (2mks)
(b) Write an ionic equation for the reaction. (1mk)
(c) Explain why iodine sublimes when heated to form a purple vapour. (1mk)
13. The set-up below was used to investigate the products of burning methane gas. Study it and answer the questions that follow:
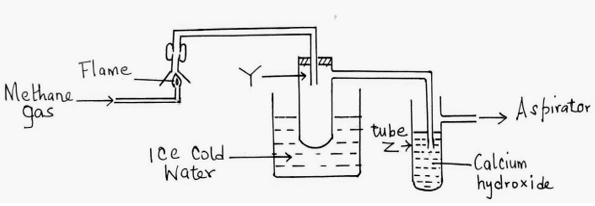
(a) What product will be formed in the test tube Y? (1mk)
(b) State and explain the observations made in tube Z. (2mks)
14. Below are PH values of some solutions.

(i) Which solution is likely to be
I Acidic rain. (1mk)
II Potassium hydroxide (1mk)
(ii) A basic substance V reacted with both solutions Y and X. What is the nature of V. (1mk)
15. In cold countries, salt is sprayed on the road to melt ice but in the long run it costs the motorists.
(a) How does the salt help in melting ice? (1mk)
(b) How does the salt affect the motorists? (1mk)
16. Using dots (.) and crosses (x) to represent electrons, show bonding in the compounds formed when the following elements react: (Si=14, Na=11, Cl=17).
(a) Sodium and chlorine. (2 Mks)
(b) Silicon and chlorine. (2 Mks)
17. (a) State Graham’s law of diffusion. (1mk)
(c) 20cm³ of an unknown gas Q takes 12.6 seconds to pass through a small orifice, 10cm³ of oxygen gas takes 11.2 seconds to diffuse through the same orifice under the same conditions
of temperatures and pressure. Calculate the molecular mass of unknown gas Q (O = 16). (3mks)
18. The peaks below show the mass spectrum of element X.
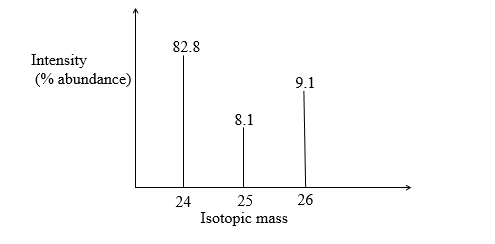
Calculate the relative atomic mass of X. (2mks)
19. Name the following compounds using the IUPAC rules.
(a) #CH_3CH_2CHCH_2CH_2CH_3#
|
#CH_2CH_3# ______________________________________________(1mk)
(b) #CH_3CHCHCH_3# ___________________________________________(1mk)
(c) Draw TWO structural formulae of isomers of compound with the molecular formula #CH_3CH_2CH_2CH_3# (2mks)
20.(a) What is meant by allotropy? (1 mk)
b) The diagram below shows the structure of one allotropes of carbon.
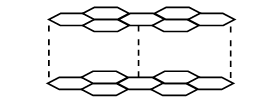
i) Identify the allotrope ( 1mk)
ii) State one property of the above allotrope and explain how it is related to its structure. (2mk).
21. 24cm³ of a solution of 0.1 M potassium hydroxide were exactly neutralized by 30cm³ of a solution of sulphuric acid. Find the molarity of the acid. (3 mks)
22.(a) Give one use of hygroscopic substances in the laboratory. (1 mk)
(b) What is meant by the terms: (2 mks)
(i) Isotopes
(ii) Mass number
(c) The formulae for a chloride of phosphorus is PCl3. What is the formula of its sulphide? (1 mk)
23. The diagram below shows the Frasch process used for extraction of sulphur.Use it to answer the questions that follow.
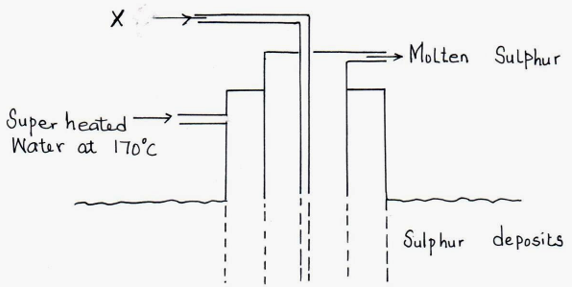
(i) Identify X. (1mk)
(ii) Why is it necessary to use super heated water in this process? (1mk)
(iii) State two physical properties of sulphur that makes it possible for it to be extracted by this method. (1mk)
24. A certain carbonate #XCO_3# ,reacts with dilute hydrochloric acid according to the equation given below:
#XCO_(3(s)) +2HCl_((aq)) rarr XCl_(2(aq)) + CO_(2(g)) + H_2O_((l))#
If 4g of the carbonate reacts completely with 40cm3 of 2M hydrochloric acid, calculate the relative atomic mass of X. (C=12.0 ,O=16.0, Cl=35.5). (3 Mks)
25. Concentrated sulphuric acid is slowly added to a mixture of freshly prepared solution of iron (II) sulphate and potassium nitrate as below.
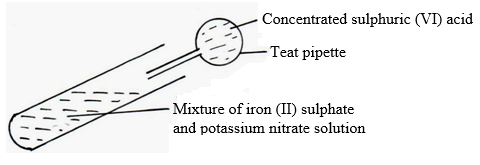
(i) State the observation made. (1mk)
26. The table below gives some properties of three substances I, J and K. Study it and answer the questions that follow.
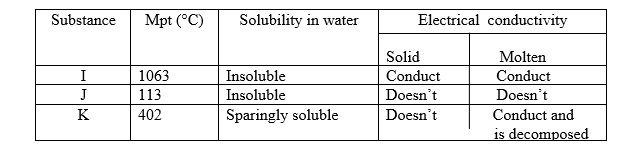
(a) Suggest the type of structure in
(i) I (1mk)
(ii) K (1mk)
(b)Explain why the molten K is decomposed by electric current but I is not decomposed.(2mks)
More Examination Papers