 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 3 Chemistry Paper 3 End of Term 3 Exams 2021
Class: Form 3
Subject: Chemistry
Level: High School
Exam Category: Form 3 End Term 3 Exams
Document Type: Pdf
Views: 1023
Downloads: 18
Exam Summary
CHEMISTRY FORM THREE PAPER 3 PRACTICAL(Plus confidential)
QUESTION 1 (20 MARKS)
You are provided with:
• Solution H which is potassium manganate (VII) solution.
• Solution X which is dilute solution of hydrogen peroxide.
• Solution N which is 0.02M ammonium iron (II) sulphate solution.
You are required to:
i) Standardize solution H using solution N.
ii) Use the standardized solution H to determine the concentration of solution X.
Procedure 1
1. Fill the burette with solution H.
2. Pipette #25cm^3# of solution N and transfer it into a conical flask.
3. Titrate solution N against solution H until a permanent pink colour just appears.
4. Record the results in table 1 below.
5. Repeat the titration two more times to complete the table.
a) Table 1
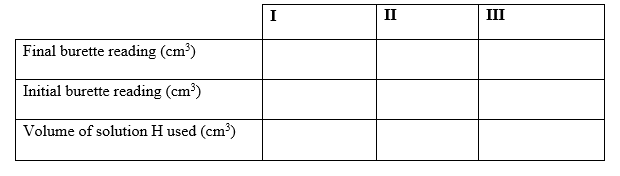 (3mks)
(3mks)
b) Determine the average volume of solution H used. (1mk)
c) Calculate;
i) The number of moles of solution N in #25cm^3#. (2mks)
ii) The number of moles of solution H that reacted given the equation for the reaction is (2mks)

iii) The concentration of H in moles per litre. (2mks)
Procedure II
1. Fill the burette with solution H.
2. Using a clean pipette, place #25cm^3# of solution X into a conical flask.
3. Add #10cm^3# of 1M sulphuric acid and shake well.
4. Titrate using solution H until a permanent pink colour just appears.
5. Record the reading in table II below.
6. Repeat the titration two more times to complete the table.
d) Table II
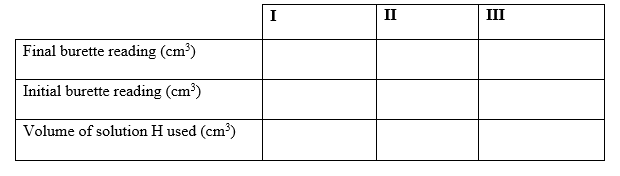 (3mks)
(3mks)
e) Determine the average volume of solution H used. (1mk)
f) Calculate;
i) The number of moles of solution H used. (2mks)
ii) The number of moles of solution X in #25cm^3# if the equation for the reaction is; (2mks)

iii) The concentration of solution X in moles per litre. (2mks)
QUESTION 2 (11 MARKS)
You are provided with the following;
• Solid Y
• Sodium chloride solution
• Potassium chloride solution
• Calcium chloride solution
You are required to identify the cations present in solid Y.
The following notes will assist in making the correct observations and inferences.
Cations are positively charged ions, majority of which are metal ions. Cations can be tested using one or a combination of the following methods;
a) Flame tests
• Some cations burn with flames that have distinct colours.
b) Carrying out precipitation reactions using the following;
i) Sodium hydroxide
ii) Aqueous ammonia
iii) Anions such as #CO_3^(2-), SO_4^(2-), Cl^- and SO_3^(2-)#
Precipitates are formed as a result of formation of insoluble salts or metal hydroxides. The colour of the precipitate should be noted down when writing the observations. Incase a white precipitate is expected and not observed, then one should record that there is no white precipitate but NOT no observation.
It is important to note that hydroxide of zinc, lead and aluminium are amphoteric thus can react with sodium hydroxide which is alkaline. Another thing to note is that zinc hydroxide and copper (II) hydroxide dissolve in excess aqueous ammonia due to formation of complex ions.
Procedure
Carry out the tests below and record your observations and inferences in the spaces provided.
a) Place all of the solid Y provided in a boiling tube. Add about #10cm^3# of distilled water and shake well. Use about #2cm^3# of the resulting solution to carry out tests (i)to (iii) below. Reserve the remaining portion for test (b).
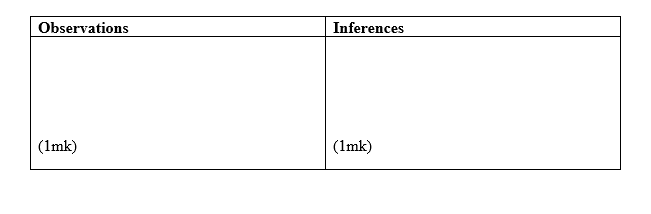
i) To the first portion, add aqueous sodium hydroxide dropwise until in excess.
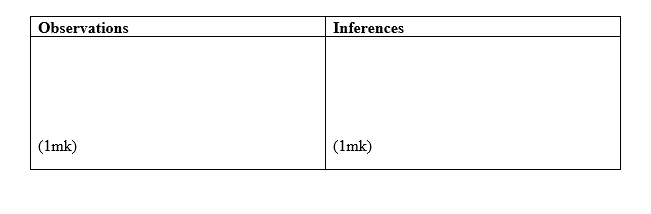
ii) To the second portion, add aqueous ammonia dropwise until in excess.
iii) To the third portion, add about #1cm^3# sodium chloride solution.
b) Procedure
Clean a glass rod and rinse it with distilled water. Dry the glass rod on a Bunsen burner flame. Allow it to cool. Dip it in a little sodium chloride solution and burn it strongly with a non-luminous Bunsen burner flame. Note the colour of the flame and record it in table III below. Clean the spatula thoroughly and repeat the procedure using each of the other solutions and complete table III.
i) Table III
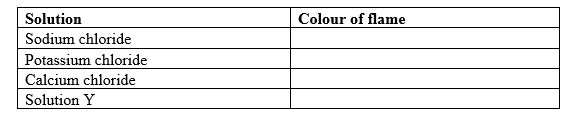
ii) From table III, suggest the cation that could be present in solid Y____________________________ (1mk)
QUESTION 3 (9 MARKS)
You are provided with solid F.
Procedure
Carry out the tests below using solid F. write the observations and inferences in the spaces provided.
a) Place all solid F in a dry boiling tube. Add about #15cm^3# of distilled water and shake thoroughly. Use #2cm^3# portions of the solution for tests (b) to (e) below.
b) To the first portion add two drops of universal indicator and record the color and PH.
c) To the second portion add a spatula and full of sodium carbonate.
d) To the third portion add two drops of bromine water.
e) To the fourth add three drops of acidified potassium manganate (VII)
More Examination Papers