 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 3 Chemistry Paper 2 End of Term 3 Exams 2021
Class: Form 3
Subject: Chemistry
Level: High School
Exam Category: Form 3 End Term 3 Exams
Document Type: Pdf
Views: 1003
Downloads: 28
Exam Summary
1. A compound D of molar mass 42 was found to have the following composition by mass Carbon = 85.7 %, and the rest hydrogen (C=12, H=1)
a) Find the molecular formula of the compound (3mks)
b) Draw the structural formula of compound D (1mk)
c) Name the homologous series to which compound D belongs (1mk)
d) About #5cm^3# of compound D was bubbled into a boiling tube containing hydrogen bromide The mixture was shaken and then allowed to stand for about 2 hours A new compound K was formed Name and draw the structural formula of compound K (2mks)
e) Give two functions of compounds to which compound D belongs (2mks)
f) A compound X reacts with bromine to form another compound W whose structural formula is as follows: -
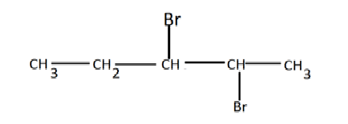
(i) Name compound X and W (1mk)
X
W
(ii) What observation is made in this reaction? (1mk)
(iii) Write a balanced equation for the reaction between X and bromine (1mk)
2. a) The scheme below shows various reactions starting with ammonia Study it and answer the questions that follow
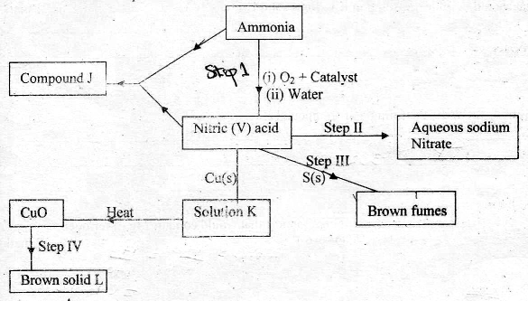
(i) Name the raw materials used in the manufacture of ammonia ( 2 mks )
(ii) Name a catalyst that is used in step I ? ( 1 mk )
(iii) Write an equation for the reaction that occurs between ammonia and nitric (V) acid ( 1 mk )
(iv) Identify the process in step II ( 1 mk )
(v) State the condition necessary for the reaction in step III to occur ( 1 mk )
(vi) Name solution K ( 1 mk )
(vii) Write the formula of solid L ( 1 mk )
(viii) Calculate the mass of solid L that would be formed by using 12 #dm^3# of hydrogen at room temperature in step IV.(Molar gas volume at rtp = 24 #dm^3#, RAM of L = 63.5) ( 3mks)
(b) (i) Identify the brown fumes in Step III (1mk)
(ii) State the property of nitric(V) acid demonstrated by the reaction in Step III above (1mk)
3 A form three student was provided with the following reagents:
(i) Solid L; containing 5.0g per litre of a dibasic organic acid #H_2X2H_2O#
(ii) Solution M; which is acidified Potassium manganate(VII)
He was required to standardize solution M using solution L. A burette was filled with solution M and #250cm^3#of solution L was pipetted into a conical flask It was then heated to about 70°C, the hot solution L was titrated with solution M while shaking the flask thoroughly until a permanent pink colour just appeared The procedure was repeated two more times and the results entered in the table1 below.
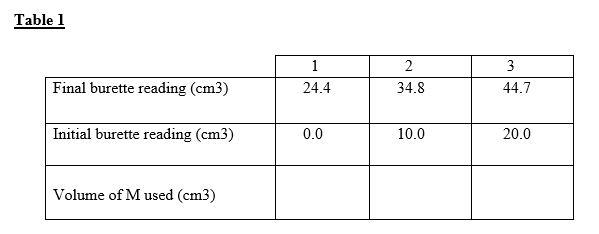
(a) Complete the table (3mks)
(b) Calculate the average volume of solution L used (1mk)
(c) Given that the concentration of the dibasic acid is 0.05M, determine the value of X in the formula #H_2X2H_2O# (H=1.0,O=16.0) (2mks)
(d) Calculate the number of moles of the dibasic acid #H_2X2H_2O# used (1mk)
(e) Given the mole ratio manganate(VII)#(MnO_4^-)#: acid #H_2X# is 2:5, calculate the number of moles of manganate(VII) #(MnO_4^-)# in the average titre (2mks)
(f) Calculate the concentration of the manganate(VII), #(MnO_4^-)# in moles per litre (2mks)
4. Dry hydrogen chloride gas was passed through heated iron wire as shown in the diagram below
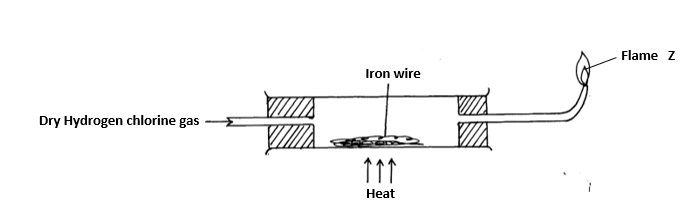
a) (i) How can the identity of the substance burning with flame Z be confirmed (1mk)
(ii) What is observed in combustion tube during the experiment? (1mk)
(iii) Write the equation for the reaction taking place in the combustion tube (1mk)
(iv) Chlorine gas was passed over the product obtained in the combustion tube to give another product Q
a) State one precaution that should be taken Explain (2marks)
b) Identify product Q (1mark)
c) The total mass of product Q formed was found to be 5.3g Calculate the volume of chlorine gas used(Cl = 35.5, Fe= 56, Molar gas volume at room temperature = 24000#cm^3# (3marks)
d) Chlorine bleaches by oxidation while Sulphur (IV)oxide does so by reduction Explain (2 mks)
5 a) What name is given to a compound that contains carbon and hydrogen only? (1mk)
b) Hexane is a compound containing carbon and hydrogen
i) What method is used to obtain hexane from crude oil? (1 mk)
ii) State one use of hexane (1 mk)
c) Study the flow chart below and answer the questions that follow
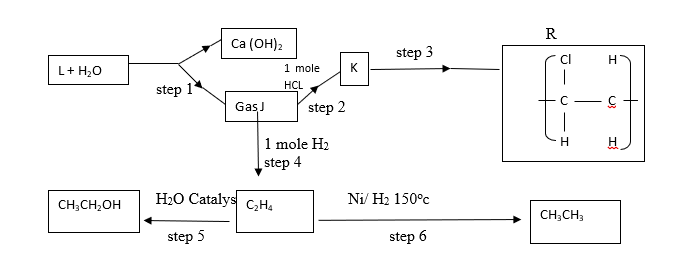
i) Identify reagent L (1 mk)
ii) Name the catalyst used in step 5 (1 mk)
iii) Draw the structural formula of J (1 mk)
iv) What name is given to the process that takes place in step 5? (1 mk)
v) State;
a) One use of product R (1 mk)
b) A commercial application of the process which takes in step 6 (1 mk)
vi) #30cm^3# of ethene gas was exploded with #100cm^3# of oxygen gas. Determine the volume of carbon(IV)oxide gas generated (2mks)
6. Study the periodic grid below and answer the questions which follow The letters do not represent actual symbols of the elements
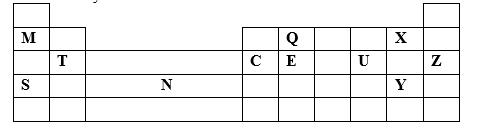
(i) To which category of elements does element N belong? (1mk)
(ii) Compare the atomic radius of element E and U Explain (2mks)
(iii) An ion #A^(3-)# has a configuration of 2.8 Place element A on the grid above (1mk)
(iv) Which of the group 1 elements will require the greatest amount of energy to remove the outermost electron Explain (2mks)
(v) Why is element Z used in light bulbs? (1mk)
(vi) Write the formula of the phosphate of element T (1mk)
(vii) State the type of bond and structure found in the oxide of element Q (2 mks)
(viii) Select from the grid:
The most electropositive element (1mk)
The most electronegative element (1mk)
7. In the preparation of magnesium carbonate, magnesium was burnt in air and the product collected Dilute sulphuric acid was added and the mixture filtered and cooled Sodium carbonate was added to the filtrate and the content filtered The residue was washed and dried to give a white powder
a) Give the chemical name of the product formed when magnesium burns in air (1mk)
b) Write a chemical equation for the formation of product in (a) above (1mk)
c) Name filtrate collected after sodium carbonate was added (1mk)
d) Write a chemical equation for the reaction between product in (a) and acid (1mk)
e) Write an ionic equation to show the formation of the white powder (1mk)
f) Write an equation to show what happened when white powder is strongly heated (1mk)
g) Study the set-up below and answer the questions that follow:
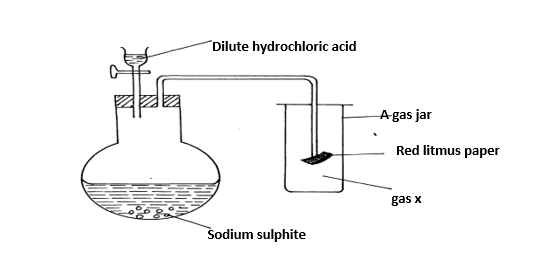
i) Identify gas X (1mk)
ii) Write an equation for the reaction that produces gas x (1mk)
iii) What is the effect of the gas X above on the red-litmus paper? (1mk
iv) What observation is made when gas X is bubbled through lead(ii)nitrate solution Explain (2 marks)
More Examination Papers