 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) Ethanol can be manufactured from ethene and steam as shown in the equation below:
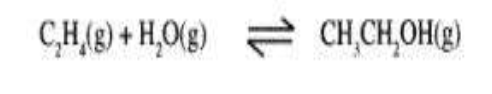
Temperature and pressure will affect the position of equilibrium of the above reaction. Name the other factor that will affect the position of equilibrium of the above reaction.
(b) The data in the table below was recorded when one mole of ethene was reacted with excess steam.
The amount of ethanol in the equilibrium mixture was recorded under different conditions of temperature and pressure. Use the data to answer the questions that follow.
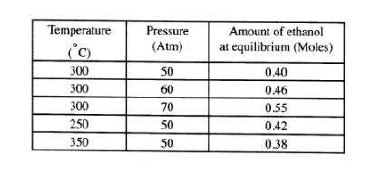
(i) State whether the reaction between ethene and steam is exothermic or endothermic. Explain your answer.
(ii) State and explain one advantage and one disadvantage of using extremely high pressure in this reaction.
I. Advantage
II. Disadvantage