 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
The curves shown below were obtained when two equal volumes of hydrogen peroxide of the same
concentration were allowed to decompose separately.
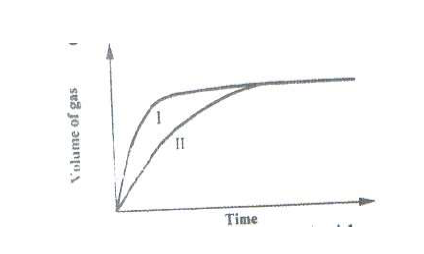
In one case, manganese (IV) oxide was added to the hydrogen peroxide. Which curve represents the decomposition of hydrogen peroxide with manganese (IV) oxide? Explain.