 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
The table below shows the first ionization energies of element B and C.
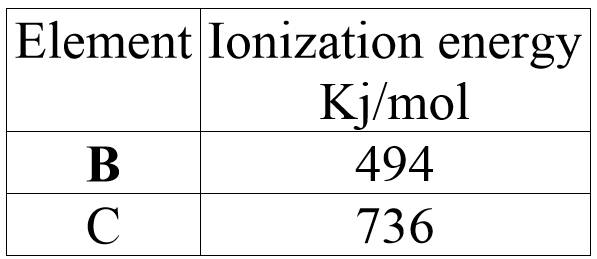
What do these values suggest about the reactivity of B compared to that of C