 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Study the information in the table below and answer the questions that follow. The letters do not represent the symbols of the elements.
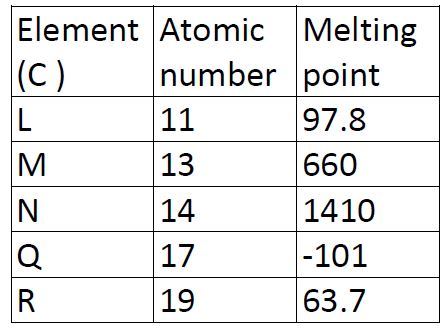
(a)Write the electron arrangement for the ions formed by elements M and Q
(b)Select an element which is
(i) the most reactive non-metal
(ii) a poor conductor of electricity
(c ) In which period of the periodic table does element R belong?
(d) Element R loses its outermost electron more readily than L. Explain.
(e ) Using dots (.) and crosses (X) to represent
outermost electrons, show bonding in the compound formed between elements N and Q.
(f) Explain why the melting point of M is higher than that of element L.
(g) Write an equation for the reaction that would occur between L and water.
(h) Describe how a solid mixture of sulphate of R and lead sulphate can be separated into solid samples.