 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 3 Chemistry Paper 2 End of Term 3 Examination 2022
Class: Form 3
Subject: Chemistry
Level: High School
Exam Category: Form 3 End Term 3 Exams
Document Type: Pdf
Views: 702
Downloads: 53
Exam Summary
Term 3 - 2022
CHEMISTRY (QUESTION PAPER)
FORM THREE (3)
Time: 2 Hours
PAPER 2
Name: …………………………………………………………. AdmNo: ……………….
School: ……………………………………………………….. Class: …………………..
Signature: …………………………………………………….. Date: …………………...
Instructions to candidate
a) Write your name, admission number, and stream in the spaces provided.
b) Answer ALL questions in the spaces provided
c) All working MUST be clearly shown where applicable
d) KNEC mathematical tables and silent non-programmable electronic calculators may be used
e) This paper consists of 9 printed pages
f) The candidate should check the question paper to ascertain that all the pages are printed as indicated and that no question is missing
1. The grid below represents part of the periodic table. Study the information and answer the questions that follow. The letters do not represent actual symbols of elements
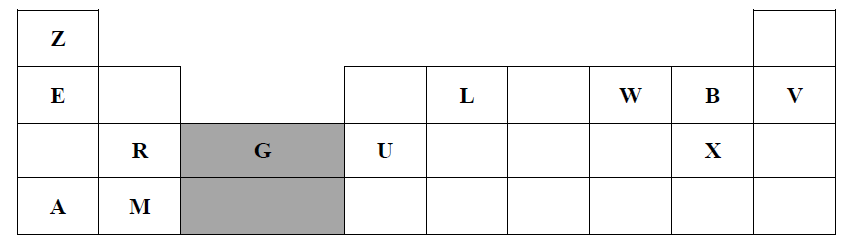
a) Which element would form a trivalent cation? (1 Mark)
___________________________________________________________________________
b) Write the equation for the reaction that would occur between R and B (1 Mark)
___________________________________________________________________________
c) What is the name of the elements that are found in the region labelled G? (1 Mark)
___________________________________________________________________________
d) Which is the most reactive non-metallic element in the table above? Explain (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
e) How does the atomic radius of L compare with that of B? Explain (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
2. The table below shows some properties and electron arrangement of ions of elements represented by letters D to K which are not actual symbols of elements. Study the information and answer the questions that follow.
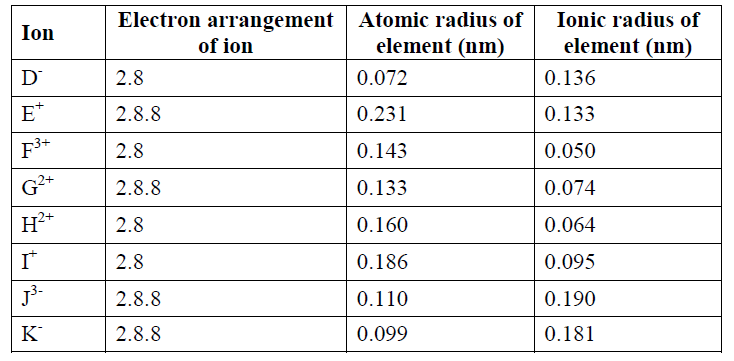
a) State the atomic numbers of elements F and G (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
b) Select two metals that belong to period 3 (1 Mark)
___________________________________________________________________________
c) Element Ireacts violently with water. Write the equation for this reaction (1 Mark)
___________________________________________________________________________
d) Why is the ionic radius of G smaller than its atomic radius? (1 Mark)
___________________________________________________________________________
e) Compare the reactivities of elements G and H. Explain (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
3. The flow chart below represents the main steps in the industrial manufacture of sodium carbonate. Study it and answer the questions that follow.
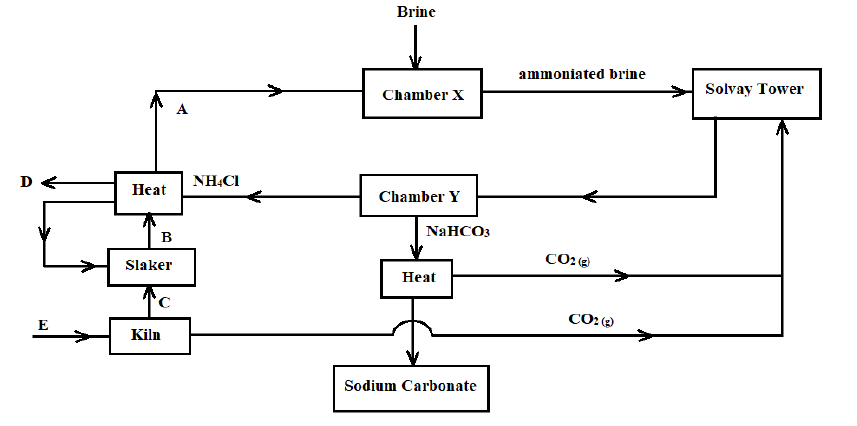
a) What is the name given to this process? (1 Mark)
___________________________________________________________________________
b) Name the substances labelled A, B, C, and D. (2 Marks)
A ________________________________ B ______________________________________
C ________________________________ D ______________________________________
c) Identify substance E and write a chemical equation for the process it undergoes to produce C (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
d) Name the process that takes place in the chamber marked Y (1 Mark)
___________________________________________________________________________
e) The process taking place in the chamber marked X requires the industry to be located near a large water body. Explain (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
f) Name two by-products that are recycled in this process (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
g) Ammonia is a raw material in the industrial manufacture of sodium carbonate. Write the chemical equations for the processes taking place in the carbonator and roaster
Carbonator: _________________________________________________________ (1 Mark)
Roaster: ____________________________________________________________ (1 Mark)
h) Considering the two reactions, and assuming that there was no recycling process, calculate the volume of ammonia at s.t.p. that would be required to produce 10.6kg of sodium carbonate if the factory is operating at 80% efficiency (3 Marks)
(C = 12.0, O = 16.0, H = 1.0, Na = 23.0, N = 14.0, molar gas volume at s.t.p. is 22.4dm3)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
i) Sodium carbonate may be prepared using the double salt, trona (Na2CO3•NaHCO3•2H2O). Why is it difficult to show that trona contains water of crystallization by heating? (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
4. Study the flow chart below and use it to answer the questions that follow.
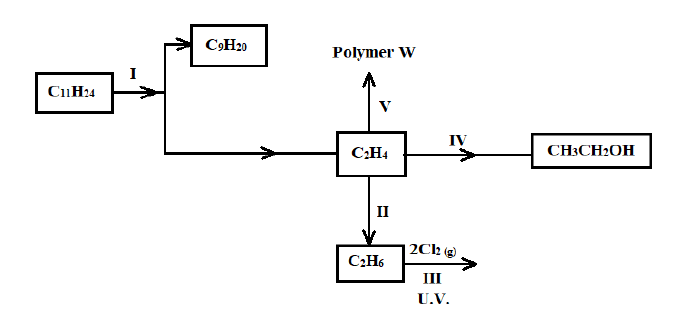
a) Name the process labelled I (1 Mark)
___________________________________________________________________________
b) Describe how acidified potassium manganate (VII) solution can be used to distinguish between C2H4 and C2H6. (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
c) State one industrial application of the process in step II (1 Mark)
___________________________________________________________________________
d) Write an equation for the reaction in step III (1 Mark)
___________________________________________________________________________
e) The compound C10H22 is cracked to obtain an alkane X and another hydrocarbon. The cracking takes place according to the following equation
C10H22-> C5H10 + X
i). Write down the formula of substance X (1 Mark)
________________________________________________________________________
ii). The cracking process requires the use of a catalyst. State two reasons why a catalyst is used in this reaction (2 Marks)
________________________________________________________________________
________________________________________________________________________
iii). Draw and name the compound C5H10 (2 Marks)
________________________________________________________________________
________________________________________________________________________
iv). Name the type of reaction that takes place when C5H10reacts with chlorine gas(1 Mark)
________________________________________________________________________
v). Draw the structure of the main product of this reaction (1 Mark)
________________________________________________________________________
________________________________________________________________________
f) What are structural isomers? (1 Mark)
___________________________________________________________________________
g) Draw and name any two structural isomers of C5H10 (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
5. The flow diagram below shows the process of obtaining nitrogen from a sample of air. Use it to answer the questions that follow.
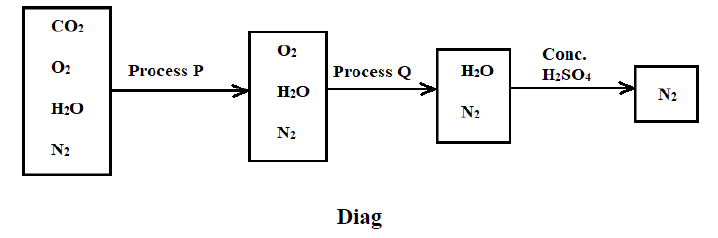
a) What is the purpose of processes P and Q? (2 Marks)
Process P ___________________________________________________________________
Process Q __________________________________________________________________
b) Identify the reagents used in the processes P and Q (2 Marks)
Process P ___________________________________________________________________
Process Q __________________________________________________________________
c) Write equations for the reactions taking place during processes P and Q (2 Marks)
Process P ___________________________________________________________________
Process Q __________________________________________________________________
d) Comment on the purity of the nitrogen gas collected (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
e) A student categorised air as a compound and not as a mixture. Give two reasons as to why the student was wrong (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
6. The diagram below illustrates an experiment setup used for the preparation of dry chlorine in the laboratory
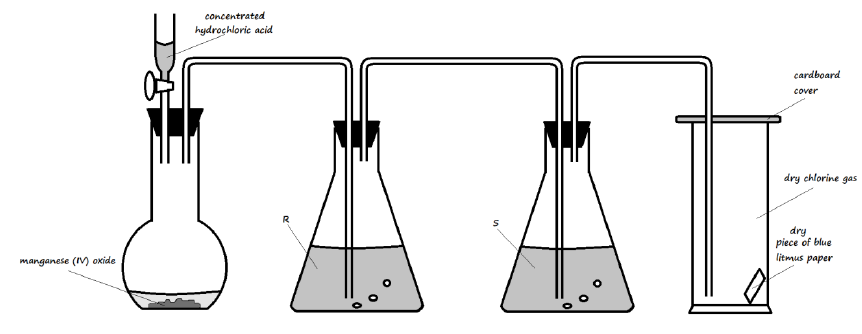
a) State one mistake that is in the setup (1 Mark)
___________________________________________________________________________
b) Write an equation for the reaction that would occur in the flask (1 Mark)
___________________________________________________________________________
c) What is the role of manganese (IV) oxide in the setup? (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
d) State the identity and roles of the following in the setup:
i). Solution R (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
ii). Solution S (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
e) State and explain one important precaution that should be observed when carrying out this experiment (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
f) State and explain the observation made on the blue litmus paper in the gas jar (2 Marks)
___________________________________________________________________________ ___________________________________________________________________________
g) State three uses of chlorine gas (3 Marks)
___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________
7.
a) Study the setup below and use it to answer the questions that follow.
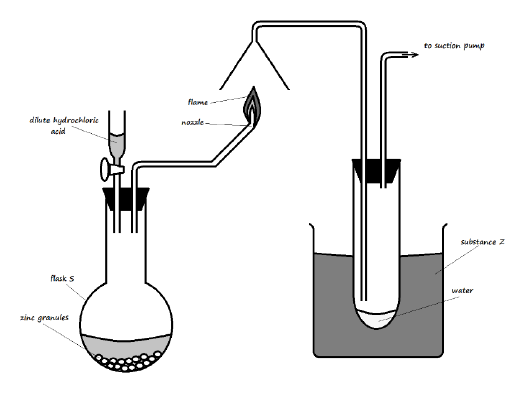
i). Identify substance Z and state its purpose in the experiment (2 Marks)
________________________________________________________________________
________________________________________________________________________
ii). Write an equation for:
The reaction in the flask (1 Mark)
________________________________________________________________________
The reaction at the nozzle (1 Mark)
________________________________________________________________________
iii). Describe a test for the product formed in flask S (2 Marks)
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
b) The diagram below was used to study a property of hydrogen gas. Study it and use it to answer the questions that follow.
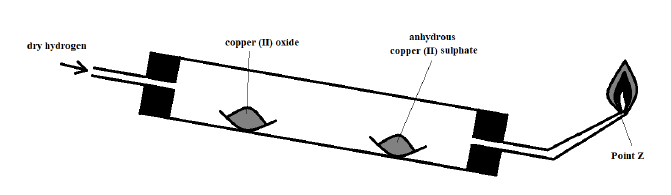
i). Name the missing condition in the setup (1 Mark)
________________________________________________________________________
ii). State two observations that may be made in the combustion tube (2 Marks)
________________________________________________________________________
________________________________________________________________________
iii). What is the importance of the reaction at the nozzle? (1 Mark)
________________________________________________________________________
________________________________________________________________________
iv). What would be observed if the copper (II) oxide were replaced with zinc oxide? (1 Mark)
________________________________________________________________________
________________________________________________________________________
v). Why is it important to tilt the combustion tube as demonstrated in the diagram? (1 Mark)
________________________________________________________________________
________________________________________________________________________
vi). State one other use of hydrogen apart from that demonstrated by the reaction in the setup (1 Mark)
________________________________________________________________________
More Examination Papers