 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 1 Chemistry Mid Term 1 Examination 2023
Class: Form 1
Subject: Chemistry
Level: High School
Exam Category: Form 1 Mid Term Exams
Document Type: Pdf
Views: 1599
Downloads: 30
Exam Summary
NAME: ………………………………… ADM NO: …………. CLASS: ………….
FORM ONE CHEMISTRY
MIDTERM EXAMS 2023
1. Define the term Chemistry. (1 mk)
2. State the major differences between the particles of solids and those of gases. (4 mks)
3. The diagram alongside shows a non-luminous Bunsen flame(burner). Study it and answer
the questions that follow. (3 mks)
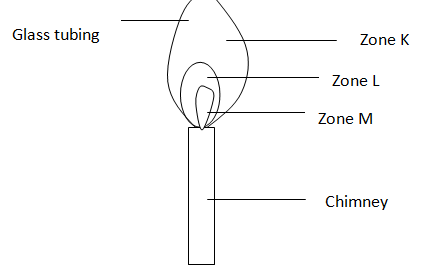
(a) Name the labeled zones based on colour
J –
K –
M –
(b) Which is the hottest part of the flame? Give a reason for your answer. (2 mks)
(c) State what would happen if a wooden alighted, splint is placed at the free end of
the glass tubing. Explain. (2 mks)
(d) Why is this flame preferred to a luminous flame for heating purposes? (1 mk)
(e) Should the air hole be open or closed to produce this flame? Explain.(2 mks)
(f) A match-stick head placed in zone M will not ignite. Explain. (2 mks)
4. Give a reason why a candle flame is not suitable for heating in the laboratory. (2 mks)
5. Besides a bunsen burner flame, name one other apparatus that can be used conveniently
for heating in the laboratory. (1 mk)
6. Draw and name 4 common apparatus used in a chemistry laboratory. (4 mks)
7. State five laboratory rules observed in a Chemistry laboratory. (5 mks)
8. Identify the processes involved in the diagram below. (2 mks)
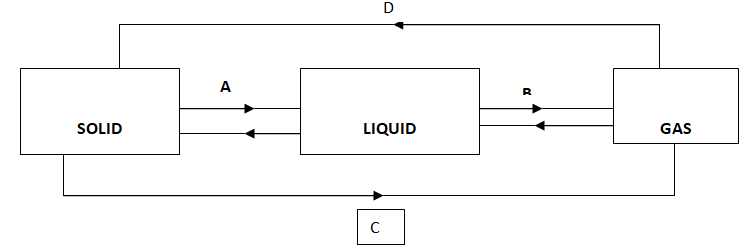
A – (½ mk)
B – (½ mk)
C – (½ mk)
D - (½ mk)
9. Name one career opportunity in Chemistry. (1 mk)
10. (a) What is drug abuse? (1 mk)
(b) What is a drug? (1 mk)
11. Explain why most laboratory apparatus are made of glass. (2 mks)
12. State four applications of paper chromatography. (4 mks)
13.The diagram below shows chromatograms for the different dyes
a) Name the techniques used to separate the dyes (1mk)
b) What conditions are required to separate the chromatograms present in a dye? (2mks)
c) What is meant by the term solvent front? Indicate the position in the diagram (1mk)
d) Which letters represent? (1mk)
i) Baseline (origin)______________
ii) Solvent path______________
e) Which chromatographs were present in dye E? (1mk)
f) Which dye is insoluble? (1/2mk)
g) Which dye is pure? Explain (1mk)
h) Which chromatogram is most soluble (1/2 mk)
14. Name two industrial application of chromatography (2mks)
15. Explain how oil would be obtained from peanuts (2mks)
More Examination Papers