 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) Below is a simplified diagram of the Downs cell used for the manufacture of sodium.
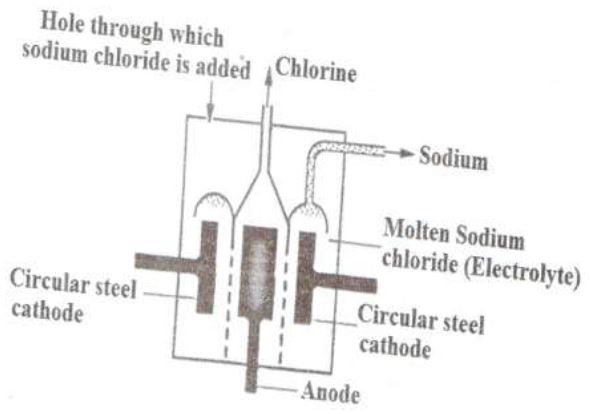
Study it and answer the questions that follow.
(i) What material is the anode made of? Give a reason.
(ii) What precaution is taken to prevent chlorine and sodium from recombining?
(iii) Write an ionic equation for the reaction in which chlorine gas is formed.
(b) In the Downs process, (used for manufacture of sodium), a certain salt is added to lower the melting point of sodium chloride from
about #800^0C# to about# 600^0C#
(i) Name the salt that is added.
(ii) State why it is necessary to lower the temperature..
(c) Explain why aqueous sodium chloride is not suitable as an electrolyte for the manufacture of sodium in the Downs process.
(d) Sodium metal reacts with air to form two oxides. Give the formulae of two oxides.