 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
(a) A student used fig 2 to investigate the action of dilute sulphuric (VI) acid on some metals. Beaker I and II contained equal
volumes of dilute sulphuric (VI) acid. To beaker I, a clean rod was dipped and to beaker II, a clean copper rod was dipped.
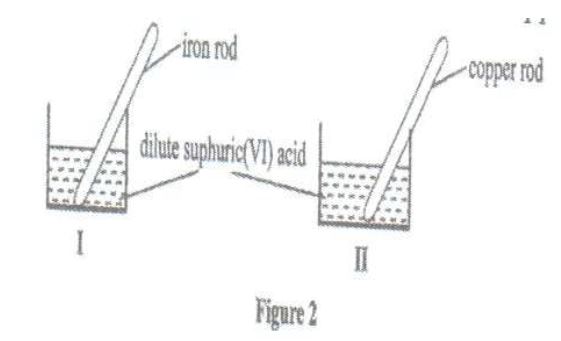
(i) Why was it necessary to clean the metal rods?
(ii) Describe the observations made in each beaker.
Beaker I
Beaker II
(iii) Explain observation in (a)(ii).
(b) Figure 3 shows the apparatus used to burn hydrogen in air. Use it to answer the questions that follow.
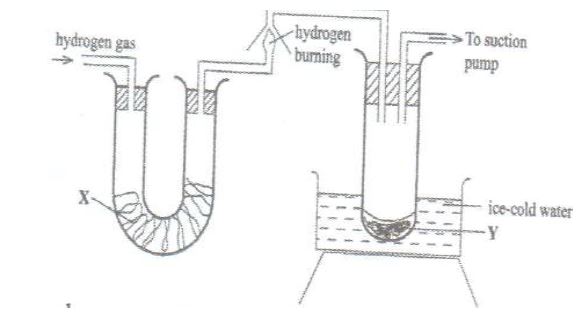
(i) State the role of substance X
(ii) Give the name of the substance that could be used as X
(iii) State the role of the suction pump
(iv) Name the product Y formed
(v) Give a simple physical test to prove identity of Y
(vi) State the difference between ‘dry’ and ‘anhydrous’