 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Study the information given below for magnesium and calcium.
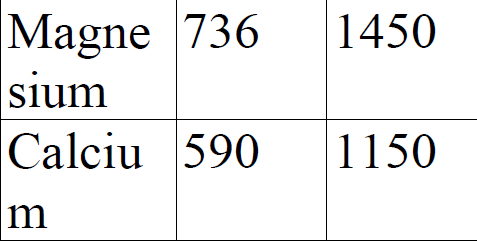
a).Explain the trend in ionization energies between magnesium and calcium.
b).Explain why the two elements have higher #2^(nd)# ionization energies than the #1^(st)# one.