 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 1 Chemistry End of Term 3 Examination 2021
Class: Form 1
Subject: Chemistry
Level: High School
Exam Category: Form 1 End Term 3 Exams
Document Type: Pdf
Views: 1799
Downloads: 76
Exam Summary
TUTORKE EXAMS
Name_______________________________________________Adm. No._________________
Class________________________ Date _____________________
FORM ONE
CHEMISTRY
TIME: 2 Hours
END TERM 3 EXAMINATIONS 2022
Instructions to Candidates
(a) Write your name and admission number.
(b) Answer ALL the questions in this question paper.
(c) All your answers must be written in the spaces provided in this question paper.
(d) Students must answer all questions in English
1. a) In the boxes provided below show how molecules are spaced in solids, liquids and gases in
terms of kinetic theory. (3mks)
b) What conclusion can you make regarding densities of solids, liquids and gases as per the
packaging of molecules in 1 (a) above. (1mk)
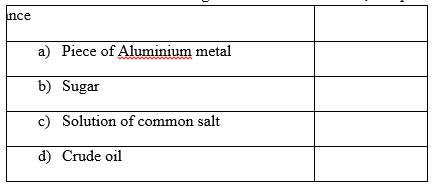
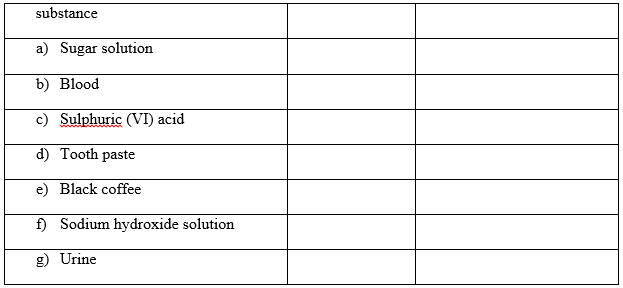
2. State whether the substances given below are elements, compounds or mixtures. (4mks)
3. Study the flow chart below and answer the questions that follows.
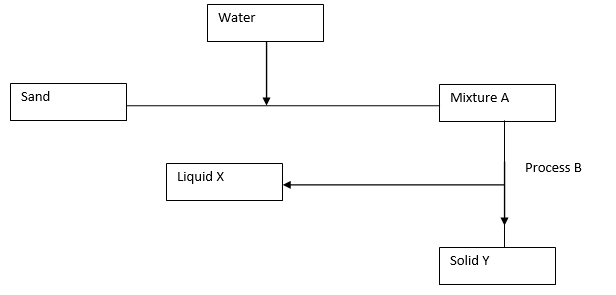
a) Name process B. (1mk)
b) Give one reason why it’s possible to separate the mixture A above using process B. (1mk)
c) Give the name for
i) Liquid X (1mk)
ii) Solid Y (1mk)
d) Give one application of process B in day to day life. (1mk)
4. State the method of separation suitable for the following mixtures.
a) Iron fillings and sulphur powder. (1mk)
b) Dye from flowers. (1mk)
c) Petrol from crude oil. (1mk)
d) Oil from nuts. (1mk)
5. The diagram below represents arrangement of particles in a substance. Study it and answer the questions that follow.
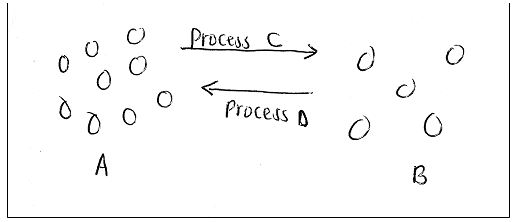
a) Name process C. (1mk)
b) Name two substances that undergo sublimation. (2mks)
c) What name is given to process D? (1mk)
6. a) If common salt is added to wax, what effect will it have on the temperature at which it melts? (1mk)
b) When alcohol is heated, it changes to gas at 780C.
i) What is the name given to this temperature? (1mk)
(ii) What will happen to this temperature if an impurity like salt is added to ethanol? (1mk)
7. Given the following substances and their PH values, indicate whether they are neutral, strongly acidic, weakly acidic, weakly alkaline or strongly alkaline. (7mks)
8. State 2 ways through which the youth of Kenya can avoid abusing drugs. (2mks)
9. A form one student at Moja High School lit a Bunsen burner with its air hole fully open.
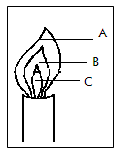
a) Which colour was the part labeled A? (1mk)
b) Identify the hottest part of the flame. (1mk)
c) Which was the almost colourless region? (1mk)
10. Classify each of the following substances as either conductors or non-conductors. (5mks)
(i) Copper metal
(ii) Paraffin
(iii) Glass
(iv) Graphite
(v) Magnesium
11. Three pure pigments were prepared and their spots placed on a filter paper as shown below. The pure pigments are A, B and C. A mixture D was also placed on the filter paper at the same time with the pure pigments.
The filter paper was then dipped in ethanol solvent and left for an hour. The results obtained were as shown below.
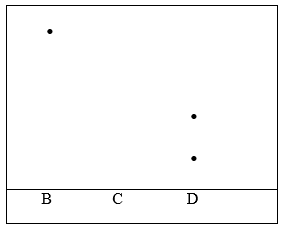
(i) Which of the three pure pigments is most sticky? Give a reason for your answer. (2mk)
(ii) Which pure pigment is not present in the mixture D? (1mk)
(iii) Show on the diagram the solvent front and the base line. (2mks)
12. a) What is an acid-base indicator? (1mk)
b) Name any three common indicators used in chemistry and give their colours in acid solution. (3mks)
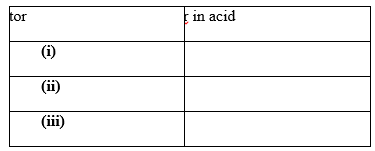
What is the advantage of universal indicator over other common acid-base indicators? (1mk)
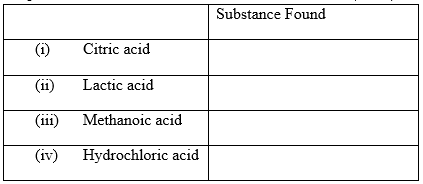
13. Citric acid, lactic acid, methanoic acid and hydrochloric acid are found in various substances in plants and animals. State where these acids occur. (4mks)
14. I. A student mixed iron fillings with sulphur powder in a watch glass. The mixture was heated and a new substance was formed.
a) Is this a physical or chemical change? (1mk)
b) Give two reasons to support your answer in (a) above. (2mks)
c) What name is given to the substance formed after heating sulphur and iron together?
(1mk)
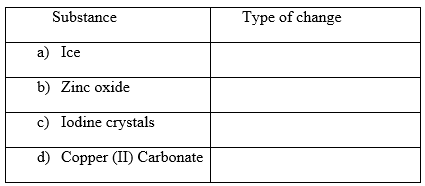
II. Determine whether the following substances undergo chemical or physical changes when heated.
(4mks)
15. Write simple word equations for the following reactions. (5mks)
(i) Magnesium and oxygen.
(ii) Carbon and oxygen (excess)
(iii) Zinc and Hydrochloric acid
(iv) Sodium Carbonate and Hydrochloric acid
(v) Calcium oxide and Sulphuric (VI) acid.
16. a) Give the chemical name of rust (1mk)
b) A form one student set up the following experiments to investigate the conditions
necessary for rusting.
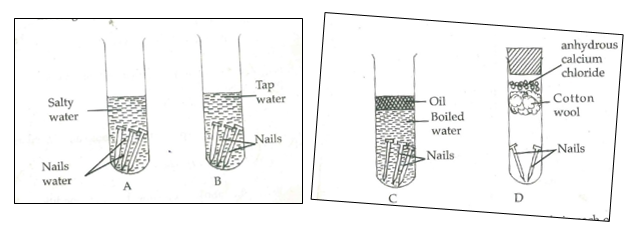
(i) What observations were made in each of the test tubes after four days. (3mks)
(ii) Why was the water in test tube C
a) Boiled (1mk)
b) Covered with oil (1mk)
(iii) What was the purpose of anhydrous calcium chloride in test tube D? (1mk)
(iv) From the above experiment, what conditions are necessary for rusting? (2mks)
(v) Name a substance that accelerates rusting. (1mk)
(vi) State 2 methods used to prevent rusting. (2 mks)
(vii) Explain why cars in Mombasa rust faster than in Nairobi. (1mk)
17. The diagram below shows the effect of heat on hydrated Copper (II) Sulphate.
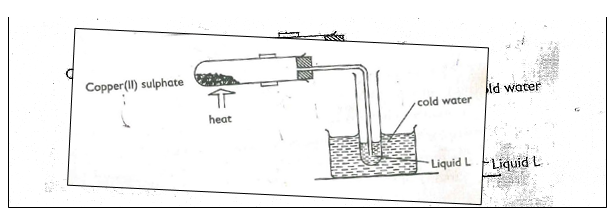
a) (i) What is the colour of hydrated Copper (II) Sulphate? (1mk)
(ii) State one observation made at the end of the experiment. (1mk)
(iii) Name liquid L. (1mk)
(iv) Name one test that can confirm the purity of liquid L. (1mk)
18. Name two apparatus that can be used to measure the volume of a gas. (2mks)
19. The table below shows liquids that are miscible and those that are immscible.
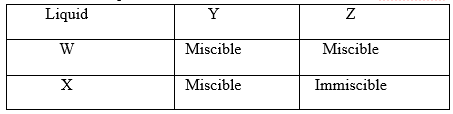
Use the above information to answer the questions that follow.
a) Name the method that can be used to separate a mixture of W and Y. (1mk)
b) Describe how a mixture of liquid X and Z can be separated. (2mks)
20. State one use of each of the following substances.
(i) Sulphuric (VI) acid. (1mk)
(ii) Magnesium hydroxide (1mk)
(iii) Nitric (V) acid. (1mk)
21. The diagram below shows preparation of oxygen gas in the laboratory.
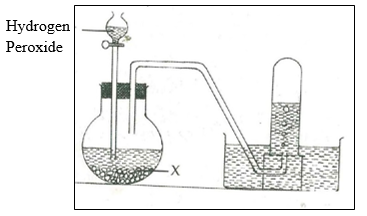
a) i) Name the reagent labeled X. (1mk)
(ii) Write a word equation for the reaction that occurs in the flask. (1mk)
b) What is the purpose of solid x in the experiment? (1mk)
c) State two physical properties of oxygen. (2mks)
d) State two uses of oxygen. (2mks)
More Examination Papers