 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 3 Chemistry Paper 1 End of Term 2 Exams 2021
Class: Form 3
Subject: Chemistry
Level: High School
Exam Category: Form 3 End Term 2 Exams
Document Type: Pdf
Views: 1019
Downloads: 40
Exam Summary
NAME………………………………………………………..…..ADM N O.……………………………….
SCHOOL…………………..…….…………DATE………………………STUDENT’S SIGN……………………....
233/1
CHEMISTRY PAPER 1
TERM TWO 2021
Time: 2 Hours
FORM THREE
INSTRUCTIONS TO CANDIDATES:
Write your name and Admission number in the spaces provided above
Answer ALL the questions in the spaces provided
Mathematical tables and electronic calculations may be used
All working MUST be clearly shown where necessary
For examiner’s use only:
TEACHER’S COMMENT ON THE SUBJECT………………………………………………………………
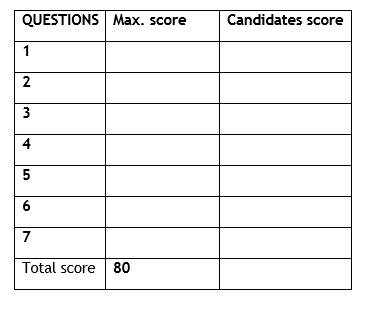
1.Identify and state the use of the apparatus shown represented below (2mks)
2.Starting with copper metal, describe how you can prepare solid copper (ii) carbonate.(3 mks)
3.When lead nitrate and sodium sulphate react, a white precipitate is formed.
i)Identify the white precipitate. (1mk)
ii) Write an ionic equation of the reaction. (1mk)
4.When anhydrous calcium chloride is exposed to the atmosphere, it behaves as shown in the equation below.
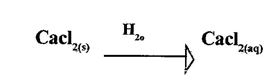
i)Name the process that takes place. (1mk)
ii)State one use of the process displayed by anhydrous Calcium chloride. (1mk)
5.a) State Graham’s law of diffusion. (1mk)
b) A given volume of carbon (ii) oxide diffuses through a hollow pipe in 30secs.Calculate the time taken by the same volume of sulphur (iv) oxide to diffuse through the same hollow pipe under the similar conditions
6.In the reaction below identify the oxidising and the reducing agents .
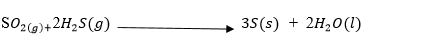
Oxidising agent___________________________________________________(1mk)
Reducing agent___________________________________________________(1mk)
7.The diagram below is a sections of a of the structure of element X
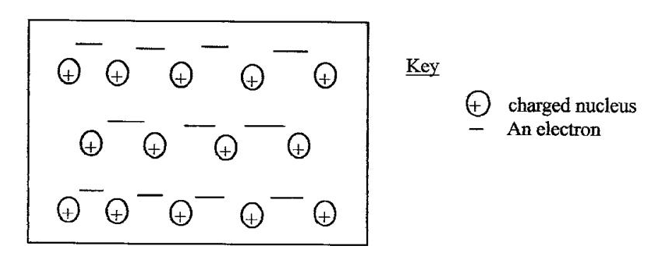
a) State the type of bonding that exists in X (1mk)
b) In which group of the periodic table does element X belong ?give a reason 2mks
8.The ionization energies for three elements A, B and C are shown in the table below.
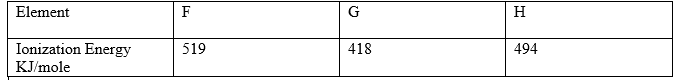
a)What is meant by Ionization energy ? (1mk)
b) Which element is the weakest reducing agent? Give a reason. (1mk)
9. 25.0cm3 of ethanoic acid ( CH3COOH ) was dissolved in water to make 500cm3 of solution.Calculate the concentration of the solution in Moles per litre. ( C = 12.0, 4 = 1.0 , 0 = 16.0, density of ethanoic acid is 1.05g / cm3 ) (3mks)
10.An element Y has a relative atomic mass of 6.939 and atomic number 3. It has two isotopes with atomic mass 6.015 and 7.016.
Calculate the relative abundances of the most abundant isotope . 2mks
11.State the functions of the following apparatus in the study of chemistry. (2mks)
a) A Desiccator
b). Pipe-day triangle
12. (a) At room temperature Silicon (iv) oxide is a solid where as Carbon (iv) oxide is a gas although Silicon is next to carbon in group (iv) of the periodic table. Explain. 2mks
b). Give one industrial use of Carbon (iv) oxide. (1mk)
13. (a) What is homologous series ? (1mk)
b) Name all the possible Isomers of an organic compound with a molecular formula C2H12.(2mks)
14. A mixture contains iron (ii) Chloride, Zinc (ii) oxide and Potassium chloride .Describe how each of the substance can be obtained from the Mixture. (2mks)
15. Give the systematic names of the following hydrocarbons :
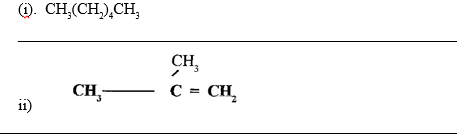
16. (a) State and explain the observation that would be made when a few drops of concentrated sulphuric Acid are added to a small sample of sugar. (2mks)
b) . Write a chemical equation for the reaction which occurs between wood and hot concentrated sulphuric (vi) acid. (1mk)
_________________________________________________________________________________
_________________________________________________________________________________
17.When lead (ii) nitrate is heated , one of the products is a brown gas .
a) Write an equation for the reaction that occurs (1mk)
b) If 0.58dm3 of the brown gas was produced ,what was the mass of the lead (ii) nitrate that was heated?
( Pb = 207,N = 14, 0 = 16, molar gas volume = 24dm3 ) (3mks)
18.The following table shows the PH values of the solutions A, B and C

i) Which solution is likely to be that of concentrated sodium chloride. (1mk)
ii) Identify the solution which is likely to be aluminium Chloride .Explain. (2mks)
19.(a) What is meant by allotropy ? (1mk)
(b). The diagram below shows the structure of one the allotropes of carbon.
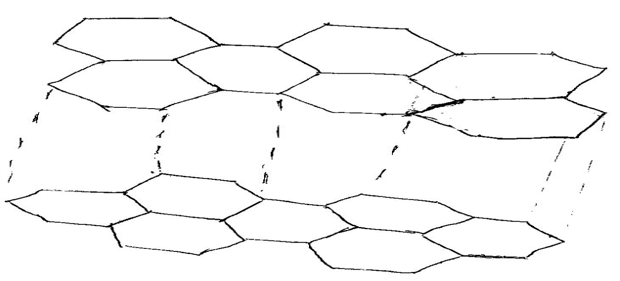
(i) Identify the allotrope. (1mk)
(ii) State one property of the above allotrope that makes it better lubricant than oil where there is a lot of friction. (1mk)
20. 0.0675 mole of a certain hydrocarbon gas on complete combustion gives 5.94g of carbon (iv) oxide and 2.43g water. Calculate its molecular formular. 3mks
21. A gas occupies 6 litres at 250k and 152 mmHg pressure. At what pressure will its volume be helved ,if the temperature then is 2270c ? 2mks
22.The table below shows some properties of three elements in group VII of the periodic table.
Study it and answer the questions that follows .
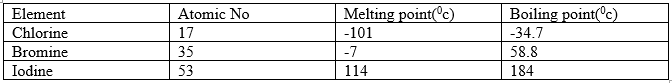
(a) Which element is a liquid at room temperature ? Give a reason 2mks
b) Explain why the boiling point of the Iodine is much higher than that of chloride. 1mk
23.The set up below was used to investigate the properties of hydrogen gas .
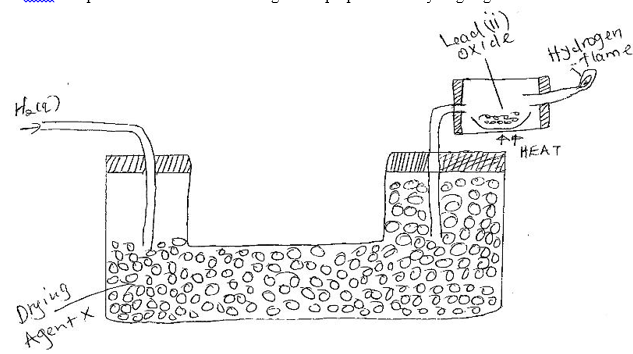
(i)Write equation for the reaction that takes place in the combustion tube and at the flame. 2mks
ii) Suggest a possible drying agent X. 1mk
24.Using dots (.) and crosses ( x) to represent electrons ,draw a diagram to represent bonding in water.
( 4 =1, 0 = 8 ). 2mks
25. 25cm3 of a solution containing 8g per litres of sodium hydroxide was neutralised by 10.0cm3 of dilute sulphuric acid in moles per litre.
(Na = 23.0, 0 = 46.0, 4 = 1.0 ) (3mks)
26.Distinguish between the terms detravescent and efforescent as used in chemistry. 2mks)
27.Carbon (iv) oxide can be dissolved in water under pressure to make an acidic solution.
a) What is meant by an acidic solution (1mk)
b)Aqueous lead (ii) nitrate reacted with the acidic solution to for a precipitate.
Write an ionic equation for the reaction. (1mk)
28.Nitrogen and Oxygen are separated from air by fractional distillation .Oxygen boils at 1880c and nitrogen at -1960c.
(a) What state must air be in before fractional distillation can be carried out
(b) Very low temperatures are required for the above process to occur. How are these achieved ) 1mk)
(c).Name one other gas that is also obtained from the fractional distillation of air. (1mk)
More Examination Papers