 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 4 Chemistry Paper 1 End of Term 2 Exams 2021
Class: Form 4
Subject: Chemistry
Level: High School
Exam Category: Form 4 End Term 2 Exams
Document Type: Pdf
Views: 862
Downloads: 29
Exam Summary
233/1
CHEMISTRY
PAPER 1
END OF TERM 2 EXAM 2021
FORM FOUR
1 [a] State Boyle’s law [1mk]
[b] At 400°C, 850#cm^3# of a gas exert a pressure of 560mmHg. What volume of the same gas would exert a pressure of 640mmHg at the same temperature? [3mks]
2. When burning magnesium is lowered into a gas jar containing nitrogen (I) oxide, it continues to burn forming a white solid
[a] Name the white solid [1mk]
[b] Write a chemical equation of the reaction that occurred [1mk]
3. Carbon {IV} oxide is one of the gases used in fire extinguishers
[a] State any other possible use of carbon {IV} oxide [1mk]
[b] Name any two reagents that can be reacted together to generate carbon {IV} oxide [2mks]
4. Rusting is a process that causes massive destruction of iron structures
[a] State one condition that accelerates rusting [1mk]
[b] State one advantage of rusting [1mk]
5. At 60°C, 38 grams of lead{II} nitrate saturate 56g of water. Determine the solubility of lead {II} nitrate at this temperature [2mks]
6. Explain why molten sodium chloride conducts electricity, but solid sodium chloride does not [2mks]
7. A polymer can be represented as
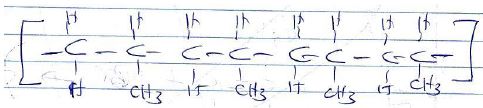
[a] Name and draw the structure of the monomer [2mks]
[b] What type of polymerization occurs in the above case? [1mk]
[c] Given that the molecular mass of the polymer is 25620, how many units of the monomer make the polymer [2mks]
8. A reaction can be represented as;

Given the bond energies of C-H, C=C, C-C, C-Br, and H –Br as 20kJ/mol, 580Kj/mole, 446Kj/mole, 438KJ/mole and 396kJ/mole respectively. Determine the heat of formation of #C_2H_5Br# [3mks]
9 [a] Define the term, dynamic equilibrium [1mks]
[b] A reaction at equilibrium can be represented as
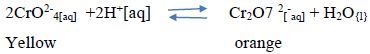
State and explain the observation made when;
[i] NaOH is added to the equilibrium mixture [2mks]
[ii] HCl is added to the equilibrium mixture [2mks]
10. During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30 minutes
[a] Write the ionic equation of the reaction that occurred at the cathode [1mk]
[b] Given R.A.M of copper = 64 and 1F = 96500C, calculate the change in mass of the cathode [3mks]
11. [a] Define the term half-life [1mk]
[b] Name two particles likely to be emitted when a radioactive nuclide undergoes radioactivity [2mks]
[c] The half-life of a radioactive nuclide is 3 hours. Given that its initial mass is 288g, determine the remaining mass after 12 hours. [2mks]
12. The reduction potentials of elements M and N are;
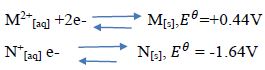
Using the above reduction potentials, predict whether a reaction would occur between #N^+#[aq] and M[[s] [3mks]
13. An hydrocarbon can be represented as: #C_2 H_2#
[a] Name the hydrocarbon [1mk]
[b] State two reagents that can be reacted together to generate the hydrocarbon [2mks]
[c] Identify the group of hydrocarbons into which #C_2H_2# belongs to [1mk]
14. [a] Name two allotropes of sulphur [2mks]
[b] In an experiment to investigate a certain property of sulphur, Maina added few drops of conc #HNO_3# to sulphur in a test tube and warmed the mixture
[i]State one observation made [1mk]
[ii]Write a chemical equation of the reaction that occurred [1mk]
15. Chlorine is commonly used in the manufacture of #Ca (OCl)_2#
[i] State one use of the above compound of chlorine [1mk]
[ii] Write a chemical equation leading to the production of #Ca (OCl)_2# [1mk]
16. A compound can be represented as
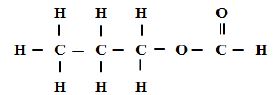
[a] What name is given to the above class of compounds [1mk]
[b] Name two reagents that can be reacted together to generate the above compound [2mks]
[c] State two conditions necessary for the reaction leading to formation of the above compound to occur [2mks]
17. Using dots and crosses, show bonding in carbon{II} oxide [2mks]
18. When 20g of a compound containing carbon, hydrogen and oxygen was burnt in the air, 29.3g of carbon{IV} oxide and 11.7g of water were produced. Determine its empirical formulae.{C=12, H=1 , O=16} [3mks]
19. Few drops of hydrochloric acid were added into a test tube containing lead {II} Nitrate solution
{a} State one observation made [1mk]
{b} Write an ionic equation of the reaction that occurred in the test tube [1mk]
20. In the industrial manufacture of Ammonia one of the raw materials is nitrogen gas
{a} Name one other raw material [1mk]
{b} Name two possible sources of the raw material you have named in {a} above [2mks]
{c} Name two substances that can be used as catalyst in this process [2mks]
{d} State one use of ammonia [1mk]
21. Gas X and Y can be collected as shown below
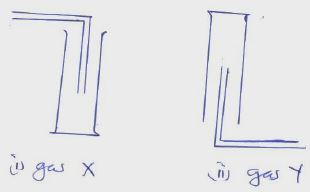
[a] Name the method used to collect gas Y [1mk]
[b] How do densities of gas X and gas Y compare? [1mk]
[c] Give an example of a gas that can be collected using the same method as gas Y [1mk]
22. Element W has two isotopes W – 36 and W-40 which occur in the ratio x:4. Given that R.A.M of W is 37.25, find the value of x [2mks]
23. Describe an experiment that can be used to determine whether a given sample of a liquid is pure [2mks]
24. A given mass of gas T diffuses through a porous plug in 48 seconds while a similar mass of gas R diffuse in 70 seconds. Given that the density of gas T is 0.6g/#cm^3#, find the density of gas R [2mks]
25. The electron configuration of elements A, B, C, D and E are as given below
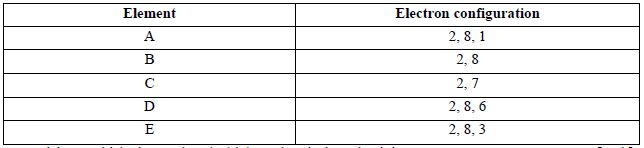
{a} Which element has the highest electrical conductivity [1mk]
{b} Which letter represents the most reactive metal [1mk]
{c} Which letter represents the most reactive non-metal [1mk]
More Examination Papers