 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 3 Chemistry Paper 1 End of Term 3 Examination 2022
Class: Form 3
Subject: Chemistry
Level: High School
Exam Category: Form 3 End Term 3 Exams
Document Type: Pdf
Views: 1002
Downloads: 89
Exam Summary
Term 3 - 2022
CHEMISTRY (QUESTION PAPER)
FORM THREE (3)
Time: 2Hours
PAPER 1
Name: …………………………………………………………. AdmNo: ……………….
School: ……………………………………………………….. Class: …………………..
Signature: …………………………………………………….. Date: …………………...
Instructions to candidate
a) Write your name, admission number, and stream in the spaces provided.
b) Answer ALL questions in the spaces provided
c) All working MUST be clearly shown where applicable
d) KNEC mathematical tables and silent non-programmable electronic calculators may be used
e) This paper consists of 10 printed pages
f) The candidate should check the question paper to ascertain that all the pages are printed as indicated and that no question is missing
1. Given a mixture of sodium chloride, silver chloride, and ammonium chloride, describe how each component can be obtained. (3 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
2. State and explain the observations made when chlorine gas is bubbled through potassium iodide solution. (2 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
3. Study the flow chart below and use it to answer the questions that follow
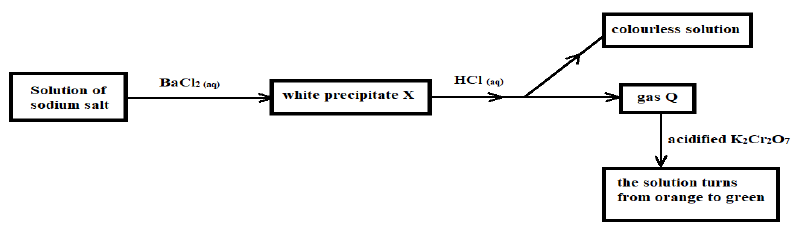
a) Name the white precipitate (1 Mark)
___________________________________________________________________________
b) Write the formula of the sodium salt (1 Mark)
___________________________________________________________________________
c) Name gas Q (1 Mark)
___________________________________________________________________________
d) Write a balanced chemical equation between aqueous hydrochloric acid and solid X(1 Mark)
___________________________________________________________________________
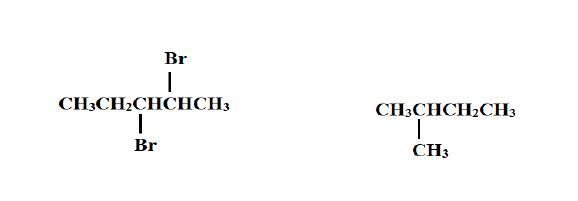
4. Give the systematic name of the following compounds (2 Marks)
5.
a) Define Gay-Lussac’s law (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
b) In an experiment a mixture of 126.0cm3 of nitrogen gas and 120.0cm3 of hydrogen gas was heated in the presence of iron catalyst. Determine the composition of the final gaseous mixture. (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
6. The table below shows certain properties of substances M, N, K, and L.
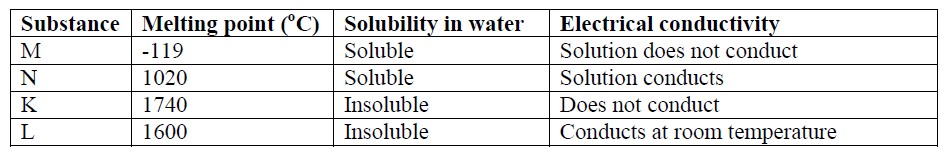
Which of the substances:
a) Is a metal (½ Mark)
___________________________________________________________________________
b) Has a simple molecular structure (½ Mark)
___________________________________________________________________________
c) Has a giant covalent structure (½ Mark)
__________________________________________________________________________
d) Has a giant ionic structure (½ Mark)
___________________________________________________________________________
7. A polymer has the following structure
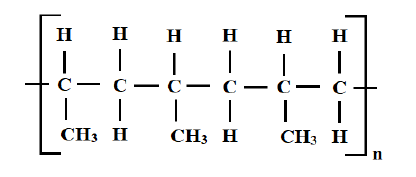
a) Draw and name the monomer (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
b) Draw the repeating unit of the polymer (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
8. Draw dot (•) and cross (×) diagrams to show bonding in:
a) Magnesium chloride (2 Marks)
b) Phophonium ion (PH4+) (2 Marks)
9.
a) A piece of burning magnesium was introduced into a jar of nitrogen. State and explain the observation made (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
b) Water was added to the product of the reaction in a) and the resultant solution tested with red and blue litmus papers. State and explain the observation made (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
10. Briefly describe how sodium carbonate powder can be obtained in the laboratory starting with concentrated sodium hydroxide solution (2 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
11. The sketch graph below shows the relationship between pressure and temperature of a gas in a fixed volume container.
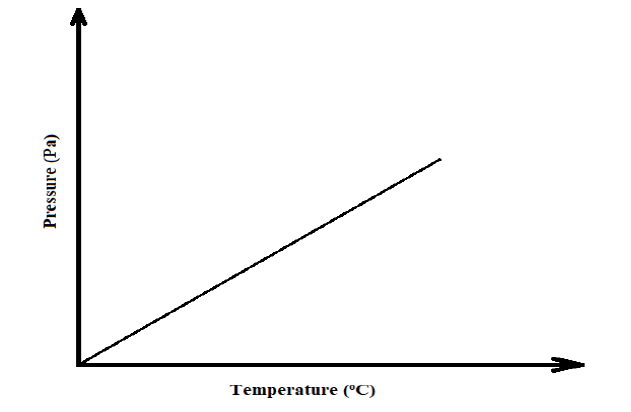
a) State the relationship between pressure and temperature that can be deduced from the graph (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
b) Using the kinetic theory of matter, explain the relationship shown by the sketch graph (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
12.
a) Group VIII elements are said to be inert. Explain. (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
b) In terms of structure and bonding, explain why group VIII elements exist as gases at room temperature (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
13. Nitric (V) acid may be prepared in the laboratory by the action of concentrated sulphuric (VI) acid in a suitable nitrate and distilling off the nitric (V) acid
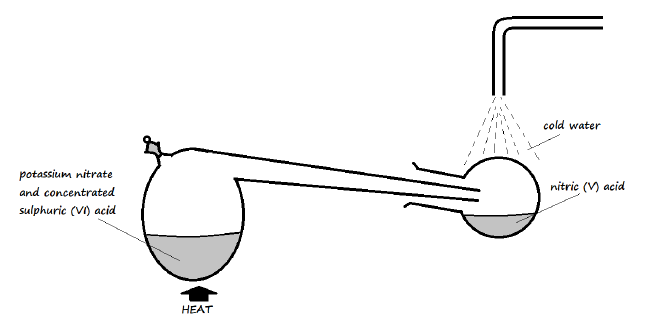
a) Why does the setup only consist of apparatus made of glass? (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
b) Pure nitric (V) acid is colourless but the product in the collection vessel is yellow. Explain (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
c) Why is it possible to separate nitric (V) acid from sulphuric (VI) acid in the setup?(1 Mark)
___________________________________________________________________________
___________________________________________________________________________
14. Name the catalyst used in the following processes:
a) Large scale manufacture of ammonia gas in the Haber process (1 Mark)
___________________________________________________________________________
b) Large scale manufacture of concentrated sulphuric (VI) acid in the Contact process (1 Mark)
___________________________________________________________________________
c) Laboratory preparation of oxygen using hydrogen peroxide (1 Mark)
___________________________________________________________________________
15. What mass of magnesium carbonate would remain if 15.0g of magnesium carbonate reacts with 25cm3 of 4M hydrochloric acid solution? (3 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
16. The setup below was used to investigate the reaction of a certain gas with lead (II) nitrate solution
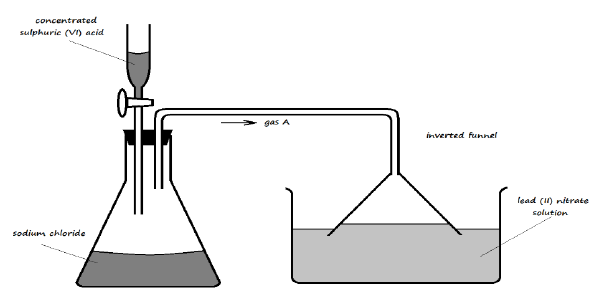
a) Identify gas A (1 Mark)
___________________________________________________________________________
b) State the observation made in the trough containing lead (II) nitrate solution (1 Mark)
___________________________________________________________________________
c) Write an ionic equation for the reaction occurring in the trough (1 Mark)
___________________________________________________________________________
17. Element Q reacts with dilute acids, but not with cold water. Element R does not react with dilute acids. Element S displaces element P from its oxide. P reacts with cold water. Arrange the four elements in order of reactivity, starting with the most reactive element. (2 Marks)
______________________________________________________________________________
______________________________________________________________________________
18. A fixed mass of a gas occupies 200cm3 at 0oC and 740mmHg pressure. Calculate its volume at -48oC and 780mmHg. (3 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
19. In an experiment, a sample of an oxide of lead was heated over coke for some time. The following results were obtained:
Mass of the oxide before heating = 8.92g
Mass of residue after heating = 8.28g
a) Determine the empirical formula of the oxide of lead (Pb = 207, O = 16) (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
b) Write an equation for the reaction in the experiment above (1 Mark)
___________________________________________________________________________
20. The information in the table below relates to the physical properties of the chlorides of certain elements.
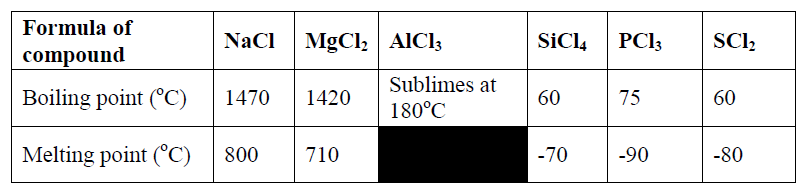
a) Select two chlorides that are liquid at room temperature (2 Marks)
___________________________________________________________________________
b) Explain why AlCl3 has a much lower melting point than MgCl2, although both aluminium and magnesium are metals. (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
21. 400cm3 of a gas D diffuses through a porous plug in 50 seconds, while 600cm3 of oxygen gas diffuses from the same apparatus in 30 seconds. Calculate the relative molecular mass of gas D. (O = 16) (2 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
22. Calculate the volume of oxygen produced when 10g of silver nitrate was completely decomposed by heating at standard temperature and pressure (Ag = 108, N = 14, O = 16, Molar gas volume at s.t.p. = 22400cm3) (3 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
23. The electron arrangement of ions W3+and Z2- are 2.8 and 2.8.8 respectively.
a) In which groups do elements W and Z belong? (1 Mark)
___________________________________________________________________________
b) Write the formula of the compound that would be formed between W and Z (1 Mark)
___________________________________________________________________________
24. 20cm3 of a solution containing 2.7g/dm3 of an alkali XOH completely reacted with 25cm3 of 0.045M sulphuric (VI) acid. Calculate the relative atomic mass of element X (O = 16, H = 1) (3 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
25. Calculate the number of sulphate ions in 150cm3 of 0.1M aluminium sulphate, Al2(SO4)3(3 Marks)
(L = 6.023 × 1023)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
26. The following apparatus was set up to investigate the percentage of oxygen in air by slowly passing 100cm3 of air from syringe A to syringe B and then back until the volume of air remained constant. Study it and use it to answer the questions that follow.
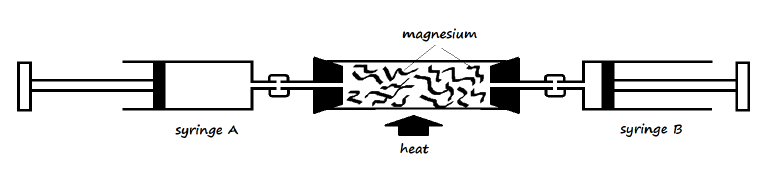
a) Identify the mistake in the setup (1 Mark)
___________________________________________________________________________
b) Why was the air moved slowly from syringe A to syringe B and vice versa? (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
c) Write an equation for the reaction that took place in the combustion tube after the mistake was corrected (1 Mark)
___________________________________________________________________________
27. A luminous flame produces bright yellow light. Explain (1 Mark)
______________________________________________________________________________
______________________________________________________________________________
28. Magnesium reacts by losing its 2 valence electrons. How does its 1st and 2nd ionization energy compare? Explain (2 Marks)
______________________________________________________________________________
______________________________________________________________________________
29. The apparatus below was used for the preparation of iron (III) chloride in the laboratory. Study it and use it to answer the questions that follow.
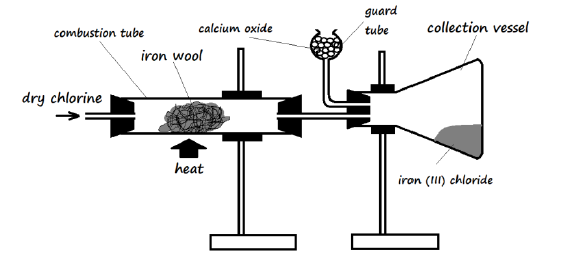
a) Why is it preferred to use calcium oxide rather than calcium chloride in the guard tube? (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
b) What property of iron (III) chloride makes it possible to be collected as shown in the diagram? (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
More Examination Papers