 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 1 Chemistry End of Term 2 Examination 2022
Class: Form 1
Subject: Chemistry
Level: High School
Exam Category: Form 1 End Term 2 Exams
Document Type: Pdf
Views: 775
Downloads: 31
Exam Summary
1. A liquid is suspected to be water by a student in the laboratory. State two chemical tests that would confirm if it is true. (2 Marks)
2. Most laboratory apparatus are made of glass. Explain. (2 Marks)
3. Nancy was stung by a bee while at school. Her Chemistry teacher applied ammonia solution to the affected area of the skin and Nancy was relieved of pain. Explain. (2 Marks)
4. Study the set-up below and answer the questions that follow
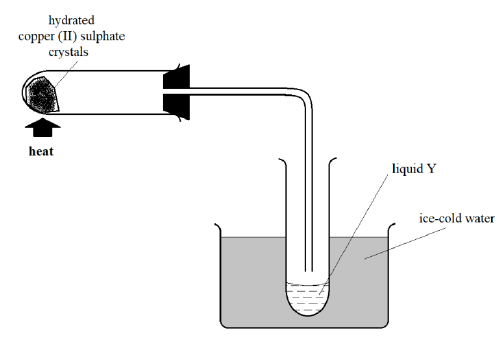
a) Give the observations made in the combustion tube during the experiment. (1 Mark)
b) What is the identity of liquid Y? _________________________________________ (1 Mark)
c) With reason, state the type of change illustrated in the experiment above. (2 Marks)
5. State and explain two factors that determine the movement of coloured pigments in a paper chromatogram. (2 Marks)
6. How does the pH value of potassium hydroxide solution compare with that of ammonia solution? Explain. (2 Marks)
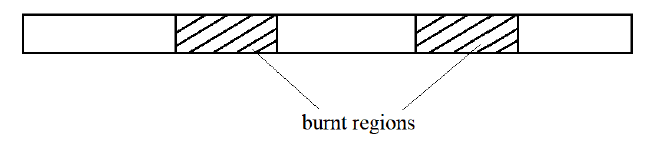
7. The figure below shows a burning splint that was put in the middle of a non-luminous flame. Explain the results. (2 Marks)
8. The pH values of solutions K, L, M, N, and P are as shown in the table below.
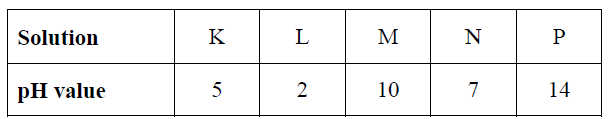
a) Define the term pH (1 Mark)
b) Identify:
i) A weak acid ______________________________________________________ (1 Mark)
ii) A strong acid _____________________________________________________ (1 Mark)
iii) A neutral solution __________________________________________________ (1 Mark)
9. Which method can be used to obtain the following? (3 Marks)
a) Salt from sea water ___________________________________________________________
b) Petrol from crude oil __________________________________________________________
c) Oil from sunflower ___________________________________________________________
10. The diagram shows spots of pure substances W, X, and Y on a chromatography paper. Spot Z is that of a mixture.
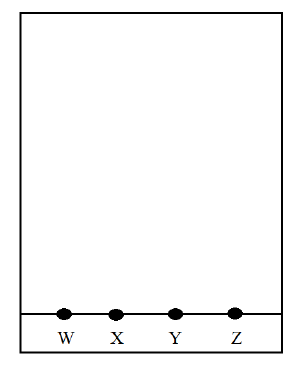
After development W, X, and Y were found to have moved 8cm, 3cm, and 6cm respectively. Z had separated into two spots which had moved 6cm and 8cm.
a) On the diagram
i) Label the baseline (1 Mark)
ii) Show the positions of all the spots after development (3 Marks)
b) Identify the substances present in mixture Z (2 Marks)
11. a) Name the elements present in the following compounds
i) Ammonium carbonate (2 Marks)
________________________________________________________________________
ii) Sodium peroxide (1 Mark)
________________________________________________________________________
iii) Potassium hydroxide (1½ Mark)
________________________________________________________________________
iv) Magnesium nitrite (1½ Mark)
________________________________________________________________________
b) Write a word equation for the reaction between solid lead (II) carbonate and dilute sulphuric acid (1 Mark)
___________________________________________________________________________
c) State and explain the observation made in the reaction above. (2 Marks)
12. Define the following terms:
a) Atom (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
b) Acid (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
c) Element (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
13. Draw the following apparatus (3 Marks)
Separating funnel
Burette
Desiccator
14. State the symbol of the following elements as used in chemistry (3 Marks)
i. Sodium __________________
ii. Zinc _______________________
iii. Cobalt ____________________
iv. Silver ___________________
v. Gold _______________________
vi. Carbon ___________________
15. Describe how solid ammonium chloride can be separated from a mixture of ammonium chloride and anhydrous calcium chloride. (2 Marks)
16. Name and draw a Bunsen burner flame that is mostly used for heating in the laboratory (4 Marks)
17. The graph below shows a curve obtained when water at 22oC was heated for 20 minutes
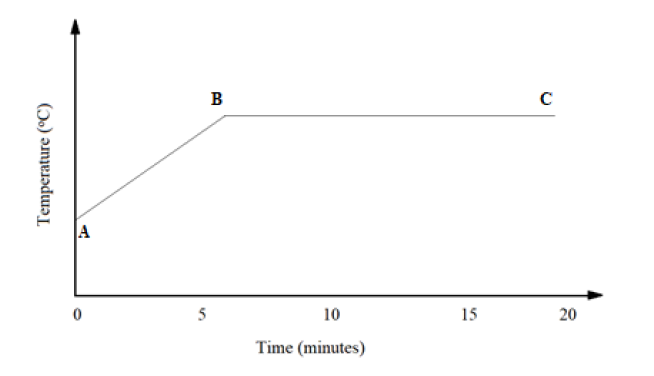
a) What happens to the water molecules between points A and B? (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
b) In which part of the curve does a change of state occur? (1 Mark)
___________________________________________________________________________
c) Explain why the temperature does not rise between B and C. (1 Mark)
___________________________________________________________________________
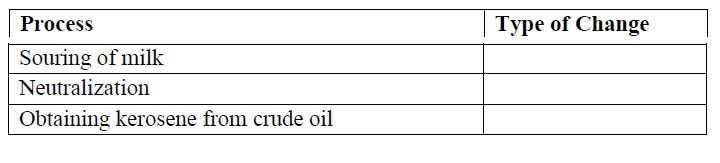
18. State the type of change that occurs during the following changes. (3 Marks)
19. State three differences between mixtures and compounds (3 Marks)
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
20. Write word equations for the reactions that involve the following substances (3 Marks)
a) Zinc and dilute hydrochloric acid
___________________________________________________________________________
b) Potassium hydroxide and sulphuric (VI) acid
___________________________________________________________________________
c) Potassium hydrogen carbonate and nitric (V) acid
___________________________________________________________________________
21. Study the table below and use it to answer the questions that follow.
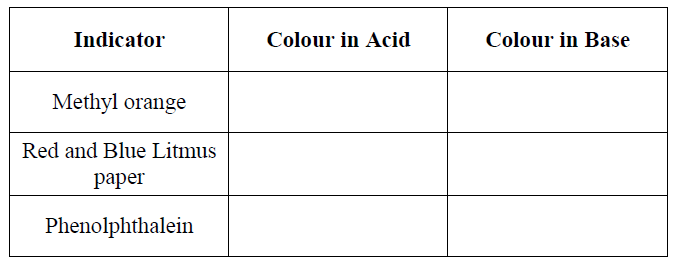
a) Complete the table above to show the colour of different indicators in acidic and basic conditions. (3 Marks)
b) State one advantage that universal indicator has over other commercial indicators (1 Mark)
22. Study the diagram below that shows a method used to separate components of a mixture. Use it to answer the questions that follow
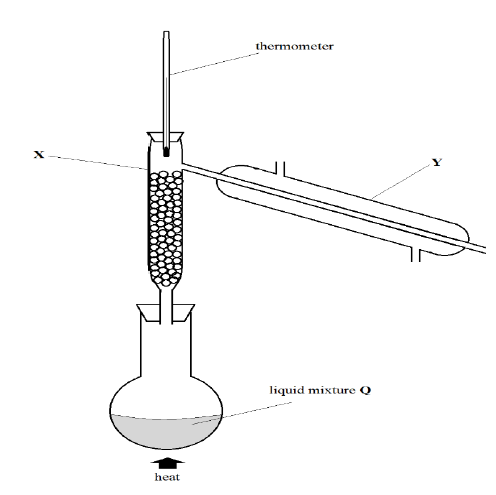
a) Name the equipment labelled X and Y (2 Marks)
X _________________________________________________________________________
Y _________________________________________________________________________
b) Show the direction of flow of water in Y (1 Mark)
c) State the physical property which allows the separation of mixtures (1 Mark)
___________________________________________________________________________
d) Give one industrial application of this method of separation of mixtures (1 Mark)
___________________________________________________________________________
e) Suppose mixture Q was paraffin and water, which apparatus would be used to separate them? (1 Mark)
___________________________________________________________________________
23. During extraction of oil from nuts:
a) Why is it important to crush the nuts? (1 Mark)
b) Why are organic solvents preferred to water in the oil extraction? (1 Mark)
___________________________________________________________________________
___________________________________________________________________________
c) Describe how can one determine that the substance produced is oil. (2 Marks)
___________________________________________________________________________
___________________________________________________________________________
More Examination Papers