 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
Form 3 Chemistry Paper 1 End of Term 3 Examination 2021
Class: Form 3
Subject: Chemistry
Level: High School
Exam Category: Form 3 End Term 3 Exams
Document Type: Pdf
Views: 1356
Downloads: 61
Exam Summary
TUTORKE EXAMS
NAME …………………………….. Candidate’s Sign. ……..…
ADM NO. ……….. Date ………………………..
FORM THREE
CHEMISTRY
END OF TERM THREE EXAMINATION 2022
PAPER – 233/1
TIME: 2 HRS
INSTRUCTIONS TO CANDIDATES
a) Write your name and admission number in the spaces provided above.
b) Sign and write the date of examination in the spaces provided.
c) ANSWER ALL QUESTIONS IN THE SPACES PROVIDED.
d) All working must be clearly shown where necessary.
e) Mathematical tables or silent electronic calculators may be used.
1. Define the following terms (3mks)
(a) Isotopes
(b)Mass number
(c) Isomers
2. (a) Give a reason why ammonia gas is highly soluble in water. (1mk)
(b) The structure of ammonium ion is shown below
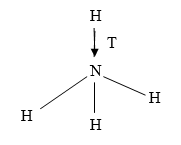
Name the type of bond represented in the diagram by ……………………… (1mk)
3. Both diamond and graphite have giant atomic structures. Explain why diamond is hard while graphite is soft. (2mks)
4. (a) Using dot (.) and crosses(x) to represent electrons, show bonding in the compounds formed when
the following elements reacts. (C-=6, Na=11, F=9)
(a) Sodium and fluorine (1mk)
(b) Carbon and fluorine (1mk)
5. The table below gives information about the major components of crude oil. Study it and answer the questions that follow.
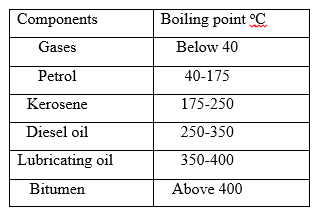
(i) Which of the compounds of crude oil has molecules with the highest number of carbon atoms?
Explain (1mk)
(ii) Name the process you would use to separate a mixture of diesel and petrol (1mk)
(iii) What condition could cause a poisonous gas to be formed when Kerosene is burnt (1mk)
6. The set up below was used to prepare nitric (V) acid
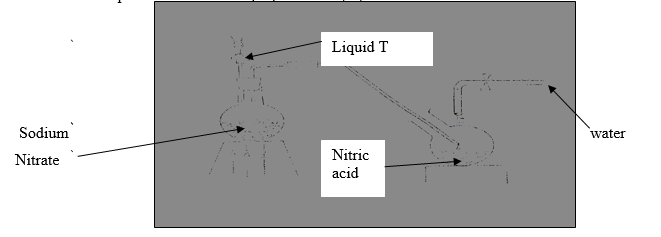
(a) Give the name of liquid T(1mk)
T……………………………… ..(1mk)
(b) Write the equation for the reaction which took place in the reaction flask (1mk)
(c ) Explain why nitric acid is stored in a dark bottle. (1mk)
7. The table below gives information on four elements represented by K L M & N. Study it and
answer the questions that follow. The letters do not represent the actual symbols of the elements.

(a) Which two elements have similar chemical properties? Explain (2mks)
8. A certain carbonate XCO3 , reacts with dilute hydrochloric acid according to the equation
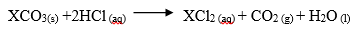
If 4g of the carbonate reacts completely with 40cm3 of 2M hydrochloric acid, calculate the relative atomic mass of X. (C=12.0 ,O=16.0, Cl=35.5). (3 Marks)
9. Give two uses of ethene gas (2Marks)
10. a) What is meant by allotropy? (1 Mark)
b) The diagram below shows the structure of one of the allotropes of carbon.
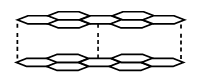
(i) Identify the allotrope ( 1 Mark)
(ii) State one property of the above allotrope and explain how it is related to its structure. (2Mark)
(iii) 60cm3 of oxygen gas diffused through a porous hole in 50seconds. How long will it take 80cm3 of sulphur(iv)oxide to diffuse through the same hole under the same conditions (S=32.0 , O=16). (3 Marks)
11. The ionization energies for three elements X,Y, and Z are shown in the table below:

(a) What is meant by ionisation energy? (1 Mark)
(b) Which element is the strongest reducing agent? Give a reason. (2 Marks)
12. In the figure below:
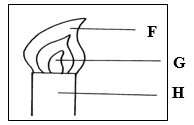
(a) Name the parts labeled F, G, and H. (1 ½mks)
F…………………………………
G…………………………………
H…………………………………
(c) Describe an experiment that would confirm that region labeled G is unsuitable for heating (1½mks)
13. The diagram below is a set up used to investigate the effect of heat on hydrated copper(II) sulphate. Study the diagram and answer the questions that follow.
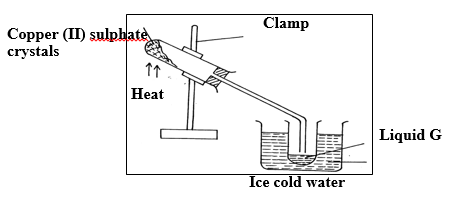
(a) Why is boiling tube slanted as shown? (1mk)
(b) What is observed in the boiling tube. (1mk)
(c) Identify liquid G. (1mk)
14. The electronic arrangement of two stable ions Q2+ and P2- are 2.8.8 and 2.8.8 respectively.
(a) Write the electron arrangement of atoms Q and P. (1mks)
Q……………………
P………………………
(b) What is the most likely structure of an oxide element P? (1mk)
15. The set up below was used by a student. Filter paper soaked in purple litmus solution was placed in the middle of the combustion tube.
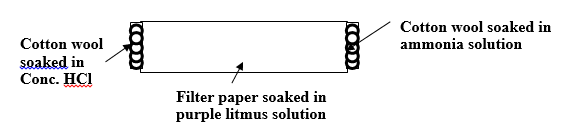
(i) What is the main aim of the experiment. (1mk)
(ii) State the first observation likely to have been made in the tube. Explain the observation. (2mks)
16. The empirical formula of a compound is CH2 and it has a molecular mass of 42.
a) What is the molecular formula of this compound? (1mk)
b) Write the general formula of the homologous series to which the compound belongs. (1mk)
c) Draw the structural formula of the third member of this series and give its IUPAC name. (1mk)
17. 3.22g of hydrated sodium sulphate, Na2SO4 X H2O were heated to a constant mass of 1.42 g. determine the value of X in the formula ( Na=23.0, S = 32.0, O = 16, H = 1) (3mks)
18. Complete the following reactions. (2mks)
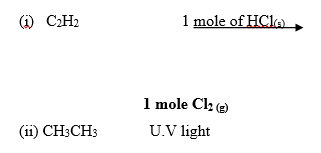
19. A white solid K was heated. It produced a brown gas A and another gas B which relights a glowing splint. The residue left was yellow even after cooling.
a) Identify gases A and B (1mks)
b) Write a balanced chemical equation for the decomposition of solid K. (1mk)
21. Explain why aluminium articles are not easily corroded. (1mk)
22. Dry chlorine gas was passed through two pieces of coloured cotton cloth as shown.
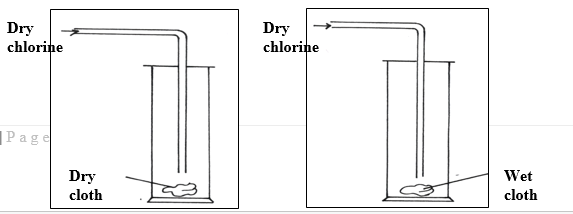
a) State what is observed in each experiment. (2mks)
Experiment 1
Experiment 2
b) Explain your observation using an equation for experiment 2 (1mk)
23. When burning magnesium ribbon is put into a gas jar of carbon (IV) oxide gas, it continues to burn
leaving behind white solid powder and black solid specks as residue write chemical equation for the
reaction that produces.
i) The white solid powder. (1mk)
ii) Black solid specks. (1mk)
24. Describe how you would obtain pure solid samples of each of the following components of a solid
mixture containing ; Lead (II) chloride, Sodium carbonate and calcium sulphate. (3mks)
25.a) State Boyle’s gas Law. (1mk)
b) A fixed mass of a gas has a volume of 250cm3 at 27oC and 750mmHg pressure. Calculate the
gas volume that the gas would occupy at 41oC and 750mmHg pressure. (0o = 273k) (2mks)
26. The diagram below shows a sample of ammonium chloride being heated in a dry boiling tube
containing a plug of cotton and dump red litmus paper
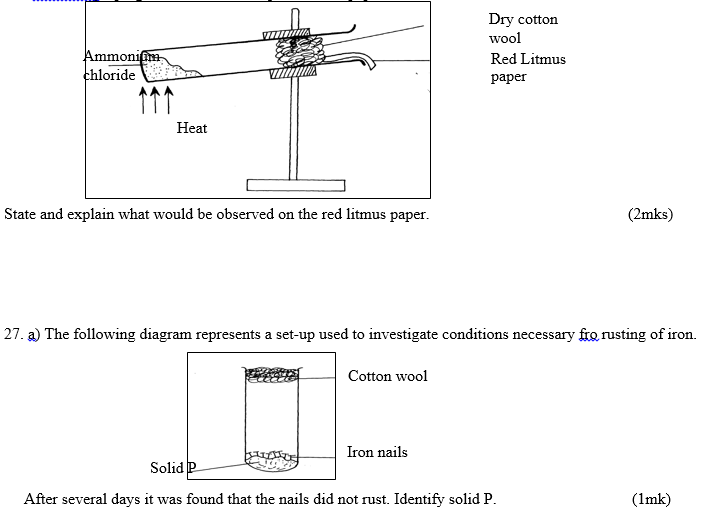
28. a) Write a chemical equation for the combustion of laboratory gas , when the Bunsen burner produces
a non-luminous flame. (1mk)
b) Describe two observable characteristics of aluminous flame. (1mk)
29 . Name the following compounds (1 mark)
(i) CH3CH=CHCH3
(ii) CH3-CH2-CH-CH2CH2CH3 (1 mark)
CH2CH3
30 . The diagram below represents aset up that can be used to react Lithium with water to produce gas X which is then reacted with copper II oxide.
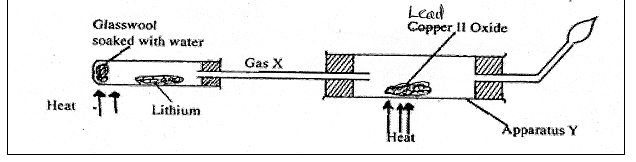
(i) Write an equation for the reaction between gas X and CuO (1mark)
(ii) Give the observation made in the apparatus (1 mark)
(iii) Why is it necessary to burn excess gas at the end of the jet (1 mark)
31.Solution R,S and T have PH values shown in the table below:
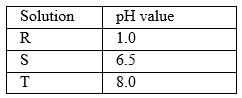
T 8.0
a) What do you deduce about the nature of solution R? (1 mark)
b) Which solution would react most vigorously with sodium hydrogen carbonate. (1 mark)
c) Which solution is likely to be ammonia solution? (1 mark)
More Examination Papers